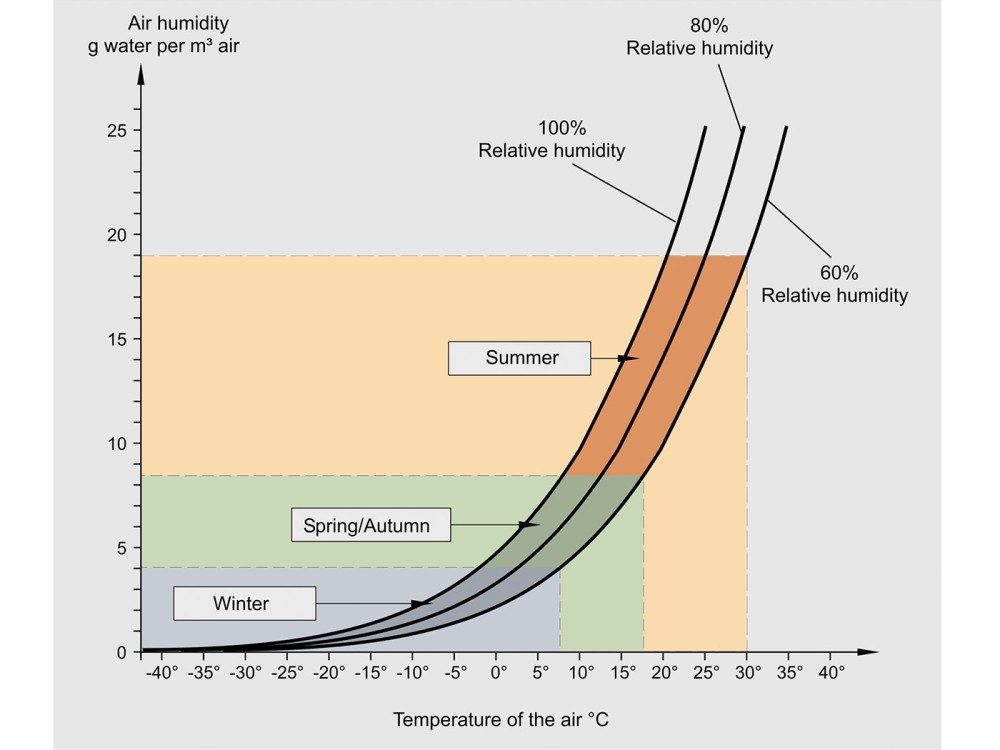
Humidity affects the dryness of the solids it is in contact with. A dynamic equilibrium is set up between the moisture in the solids and the vapour in the air. Solids left in contact with moist air for long periods will reach their equilibrium moisture content, XE , which increases as relative humidity increases or temperature falls. As a result, solids in a cool damp atmosphere will pick up more moisture than those stored in warm dry conditions. XE is often plotted as an adsorption or desorption isotherm. Equilibrium relative humidity (ERH) uses the principle in reverse.
For a given solids moisture content, the relative humidity of the air in contact with the damp solid at equilibrium will be constant. This provides a useful indirect method of measuring solids moisture content. Temperature or humidity cycling often affects solids in storage. For example, the air in an uncontrolled storage area will be at a relatively high temperature and absolute humidity during the day. At night the temperature falls and the relative humidity increases until the dewpoint is reached and air condenses on the walls and the solid surface (“silo rain”). This surface moisture then cakes the solid together into large lumps so that it will not flow freely on discharge. A similar problem can arise if solids are discharged from the dryer without cooling, and stored or bagged when hot. Further moisture is evaporated, giving high local humidity; when the air cools down to ambient, condensation again occurs. Humidity should always be carefully considered whenever there are caking and lump formation problems in storage and transport. High relative humidity may also encourage product spoilage, e.g. by promoting biological reactions and mould growth.


Comments are closed