If you have ni moles of each species (for example nA moles of A), you can try to calculate the pressure or volume that that gas alone (i.e., ignoring the other gases that are around) exerts/occupies.
DEFINITION
Partial pressure refers to the pressure that would be exerted by a species (in a mixture) if there were no other species present.
DEFINITION
The pure component volume, vA, refers to the volume that would be occupied by a species (in a mixture) if there were no other species present.
So, in an ideal gas mixture EACH COMPONENT satisfies the ideal gas law provided the partial pressure or pure component volumes are used!
PAV = nART
or
PvA = nART
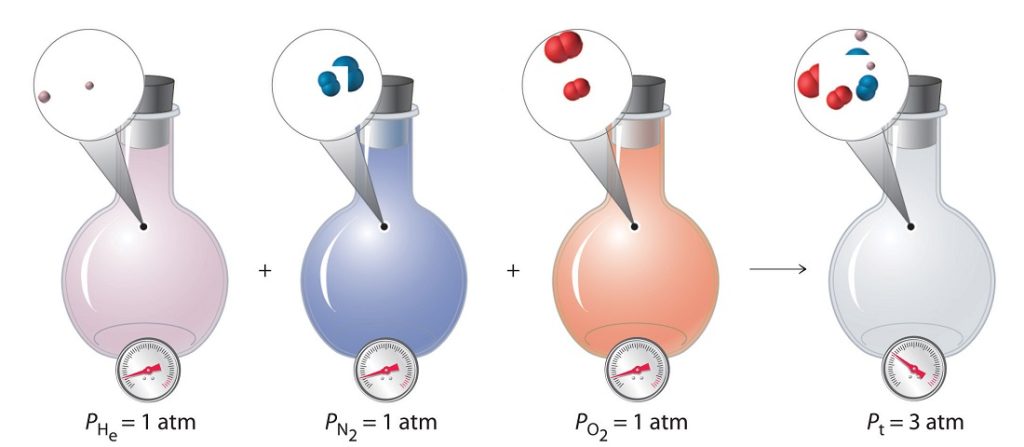
In this way, the sum of the component pressures (partial pressures) or volumes (pure component volumes) should sum to the total pressure or volume:
PA + PB + … = P
VA + VA + … = V
This is easy to see if you divide either the partial pressure equation or the pure component volume equation by the ideal gas law for the total mixture: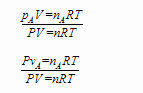
Note that RT cancels in both equations and that V cancels in the first and P cancels in the second, also that nA/n = ya. We can then rearrange the result to get:
pA = yAP
vA = yAV
So The volume fraction (or pressure fraction) of an ideal gas is equal to the mol fraction! (vA/V = nA/n)

Comments are closed