Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products that are consumed/produced within a given chemical reaction. In order to make any stoichiometric determinations, however, we must first look to a balanced chemical equation. In a balanced chemical equation, we can easily determine the stoichiometric ratio between the number of moles of reactants and the number of moles of products, because this ratio will always be a positive integer ratio. Consider the reaction of nitrogen gas and hydrogen gas to form ammonia (NH3):
[latex]N_2(g) + 3 H_2(g) \rightarrow 2 NH_3(g)[/latex]
From the balanced equation, we can see that the stoichiometric coefficient for nitrogen is 1, while for hydrogen it is 3, and for ammonia it is 2. Therefore, the stoichiometric ratio, oftentimes referred to simply as the “mole ratio” or “molar ratio,” between N2(g), H2(g), and NH3(g) is 1:3:2. In the special case where reactants are combined in their molar ratios (in this case, 1 mole of N2(g) and 3 moles of H2(g)), they will react completely with each other, and no reactant will be left over after the reaction has run to completion. However, in most real-world situations, reactants will not combine in such perfect stoichiometric amounts. In most cases, one reactant will inevitably be the first to be completely consumed in the reaction, causing the reaction to come to a halt. This reactant is known as the limiting reactant, or limiting reagent.
From this brief description, we can see that stoichiometry has many important applications. As we will see, through balancing chemical equations and determining the stoichiometric coefficients, we will be able to determine the number of moles of product(s) that can be produced in a given reaction, as well as the number of moles of reactant(s) that will be consumed. Stoichiometry can also be used to make useful determinations about limiting reactants, and to calculate the amount of excess reactant(s) left over after a given reaction has run to completion.
The Basis of Stoichiometry
The science of stoichiometry is possible because it rests upon the law of conservation of mass. Since matter can neither be created nor destroyed, nor can a chemical reaction transform one element into another element, we can be sure that the mass of each individual element present in the reactant(s) of a given reaction must necessarily be accounted for in the product(s). This physical law is what makes all stoichiometric calculations possible. However, we can only perform these calculations correctly if we have a balanced chemical equation with which to work.
Interactive: Stoichiometry and Balancing EquationsTo make hydrogen chloride or any other chemical there is only one ratio of reactants that works so that all of the hydrogen and chlorine are used to make hydrogen chloride. Try several different ratios to see which ones form a complete reaction with nothing left over. What is the simplest ratio of hydrogen to chlorine for forming hydrogen chloride?
Balancing Equations
Before performing any stoichiometric calculation, we must first have a balanced chemical equation. Take, for example, the reaction of hydrogen and oxygen gas to form liquid water:
[latex]H_2(g) + O_2(g) \rightarrow H_2O(l)[/latex]
As it is written here, we should notice that our equation is not balanced, because we have two oxygen atoms on the left side of the equation, but only one on the right. In order to balance this, we need to add a stoichiometric coefficient of 2 in front of liquid water:
[latex]H_2(g) + O_2(g) \rightarrow 2 H_2O(l)[/latex]
In doing this, however, our hydrogens have become unbalanced. To finish balancing the equation, we must add a coefficient of 2 in front of hydrogen gas:
[latex]2 H_2(g) + O_2(g) \rightarrow 2 H_2O(l)[/latex]
As we can see, the stoichiometric coefficient for any given reactant/product is the number of molecules that will participate in the reaction as written in the balanced equation. Keep in mind, however, that in our calculations, we will often be working in moles, rather than in molecules. In our example here, we can see that the stoichiometric coefficient of H2(g) is 2, while for O2(g) it is 1, and for H2O(l) it is 2. Occasionally, you might come across the term stoichiometric number, which is related to the stoichiometric coefficient, but is not the same.
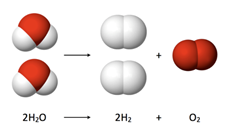
Electrolysis of waterAlthough this image illustrates the reverse reaction of [latex]2 H_2(g) + O_2(g) \rightarrow 2 H_2O(l)[/latex], the stoichiometric coefficients for each type of molecule are still the same. Water is 2, hydrogen gas is 2, and oxygen gas is 1.
For reactants, the stoichiometric number is the negative of the stoichiometric coefficient, while for products, the stoichiometric number is simply equal to the stoichiometric coefficient, remaining positive. Therefore, for our example here, the stoichiometric number for H2(g) is -2, and for O2(g) it is -1. For H2O(l), however, it is +2. This is because in this reaction, H2(g) and O2(g) are reactants that are consumed, whereas water is a product that is produced.
Lastly, you might occasionally come across some chemical species that are present during a reaction, but that are neither consumed nor produced in the reaction. A catalyst is the most familiar example of this. For such species, their stoichiometric coefficients are always zero.
Example
In the equation H2(g) + Cl2(g) → 2 HCl(g), what is the molar ratio (stoichiometric ratio) between H2(g) and HCl(g)?
In our balanced chemical equation, the coefficient for H2(g) is 1, and the coefficient for HCl(g) is 2. The molar ratio between these two compounds is therefore 1:2. This tells us that for every 1 mole of H2(g) that is consumed in the reaction, 2 moles of HCl(g) are produced.