The third law of thermodynamics is lesser known of all the three laws of thermodynamics, and even its applications found in our day-to-day life are fewer, though they can be seen in physical and chemical science at low temperatures. In simple words, the third law of thermodynamics says that “entropy of the pure crystal at absolute zero temperature is zero and entropy can never be negative.” To understand this law clearly we should know what absolute zero temperature and entropy are.
Absolute Zero Temperature
We know zero degree Celsius temperature, it is the temperature at which water gets converted into ice, and hence it is also called freezing point temperature of water. There are many gases like helium, hydrogen that can be cooled to temperatures much below zero degrees Celsius and at certain level they get liquefied. The lowest temperature, to which all the substances or gases can be cooled to, is called as absolute zero temperature. There cannot be any temperature below this point and at this temperature all the movements of all the molecules within the substance stop.
In the year 1848, Lord Kelvin devised the scale for absolute zero temperature and he concluded that absolute zero temperature meant -273.15 degree Celsius. Later, by the international agreement a new temperature scale was found by the name Kelvin. As per this scale absolute meant 0K or 0 degree Kelvin on Kelvin scale.
The relation between Kelvin and Celsius temperature scale is: K= degree Celsius + 273.
What is Entropy?
Entropy is the total energy inside the substance, which is not available for work during thermodynamic process. It can be considered as the internal energy of the substance, which depends on the movement of molecules inside the substance. The more the movement of the molecules, the more the entropy. As the temperature of the substance increases, the movement of the molecules inside the substance also increases and with it the entropy of the substance also increases.
Third Law of Thermodynamics Explained
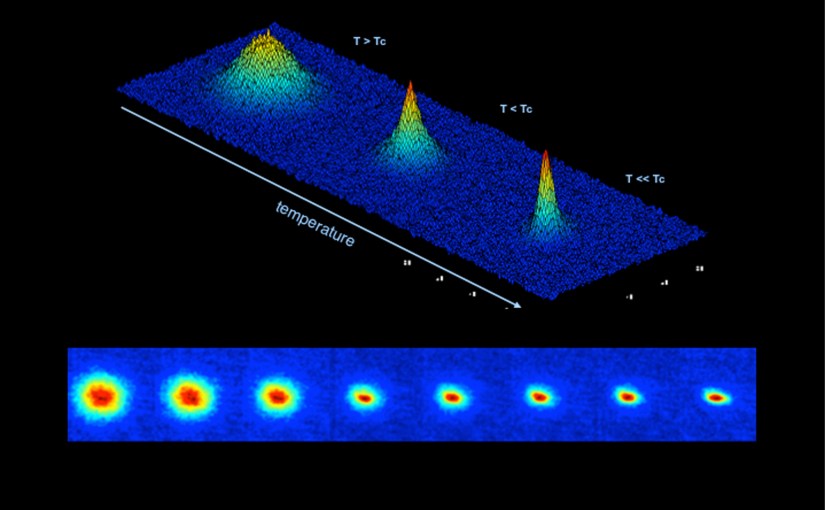
Now let us come back to third law of thermodynamics which says that at absolute zero temperature the entropy of the pure crystal is zero. A pure crystal is the substance in which all the molecules are perfectly identical and the alignment of molecules with each other is perfectly uniform throughout the substance. As per the third law of thermodynamics when such a substance is cooled to zero degree Kelvin, all the movements of all the molecules stop completely and the entropy of the substance becomes zero. This is an ideal condition.
In actuality there is no substance which has all the molecules identical and no movements of the molecules are perfectly uniform, hence in practical cases at absolute zero the entropy is not zero, its value is above zero. This also means that the value of entropy can never be negative.
Let us consider one simple example of hot steam. Steam is the gaseous form of water at high temperature. The molecules within it move freely and hence it has high entropy. If you cool this steam to below 100 degree Celsius it will get converted into water, where the movement of the molecules will be restricted resulting in decrease in entropy of water. When this liquid is further cooled to below zero degrees Celsius, it gets converted into solid ice, where the movement of molecules is further reduced and the entropy of the substance further reduces. As the temperature of this ice goes on reducing the movement of the molecules and along with it the entropy of the substance goes on reducing. When this is ice is cooled to absolute zero ideally the entropy should become zero. But in practical situations it is just not possible to cool any substance to absolute zero temperature, nor does entropy become zero, but it remains always above zero.