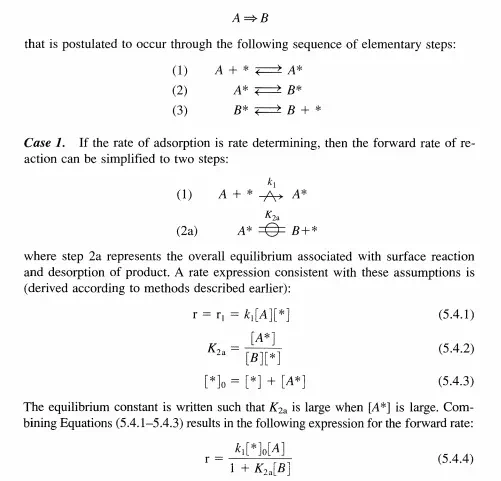
Rate data can be used to postulate a kinetic sequence for a particular catalytic reaction. The general approach is to first propose a sequence of elementary steps consistent with the stoichiometric reaction. A rate expression is derived using the steady-state approximation together with any other assumptions, like a rate-determining step, a most abundant reaction intermediate, etc. and compared to the rate data. If the functional dependence of the data is similar to the proposed rate expression, then the sequence of elementary steps is considered plausible. Otherwise, the proposed sequence is discarded and an alternative one is proposed. Consider the isomerization of molecule A far from equilibrium: