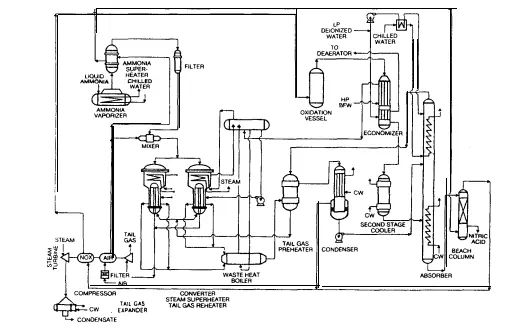
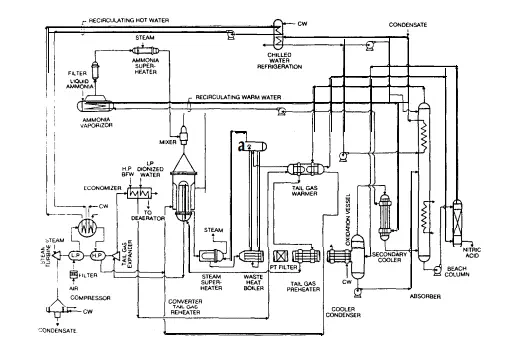
Both processes follow the basic scheme outlined in Section 1.2.4 for the catalytic oxidation of ammonia. In summary, this involves an oxidation stage whereby ammonia is reacted with air in a catalytic converter at temperatures in the range of 850-950°C. Reaction gases pass through a series of energy recovery stages before entering an absorption column. The bottoms from the column are bleached of dissolved nitrogen peroxide using air, and the resulting solution is the weak nitric acid product. The major difference between the two processes lies in the initial conversion stage.
The dual-pressure process employs a conversion stage operating in the range loo-350 kPa, and a reactor temperature of about 865°C. The single-pressure process however operates the converter at 800-l 100 kPa, with a reactor temperature closer to 940°C. Both processes are capable of highly efficient production of the same quality product with the same limits on atmospheric pollution, therefore any comparison must be related to other aspects unique to each process. These distinctions will ultimately determine the most desirable process for this application. The process comparisons are
Factors Favouring the Dual-Pressure Process
In the single-pressure and dual-pressure processes, the catalyst volatilizes at a rate determined by the converter exit-gas temperature. Experimental work indicates that the rate loss of catalyst (without a catalyst recovery system) is approximately three times more rapid at 973°C than at 866°C (Ref. PT18). From plant operation data, the loss from a dual-pressure converter (operating at 866°C) is estimated at about 0.10 g/tonne of 100% acid, and from a single-pressure converter (operating at 937°C) it is estimated at about 0.38 g/tonne of 100% acid. In the single-pressure process, the ammonia content of the mixture should not exceed 10.5% because of the lower explosive limit. In the dual-pressure process with lower converter pressures, the explosive limit is higher providing additional operating flexibility. Total production cost for a 1500 tonne/day plant built in the USA in 1979 (given in Table C.5) has a A$2.15/tonne acid advantage in favour of the dual-pressure process.
This advantage is directly attributable to a higher ammonia yield and a smaller indirect cost structure (due mainly to a 20% lower catalyst inventory requirement and less frequent catalyst changes). This production cost advantage also prevails despite inferior steam production capacity. Production cost estimates for a similar sized plant in Europe confirm the dualpressure process advantage of A$S2.00/tonne of acid produced. Although the plant data relate to overseas operations where cost structures may be different, the figures do illustrate an important and valid trend.


