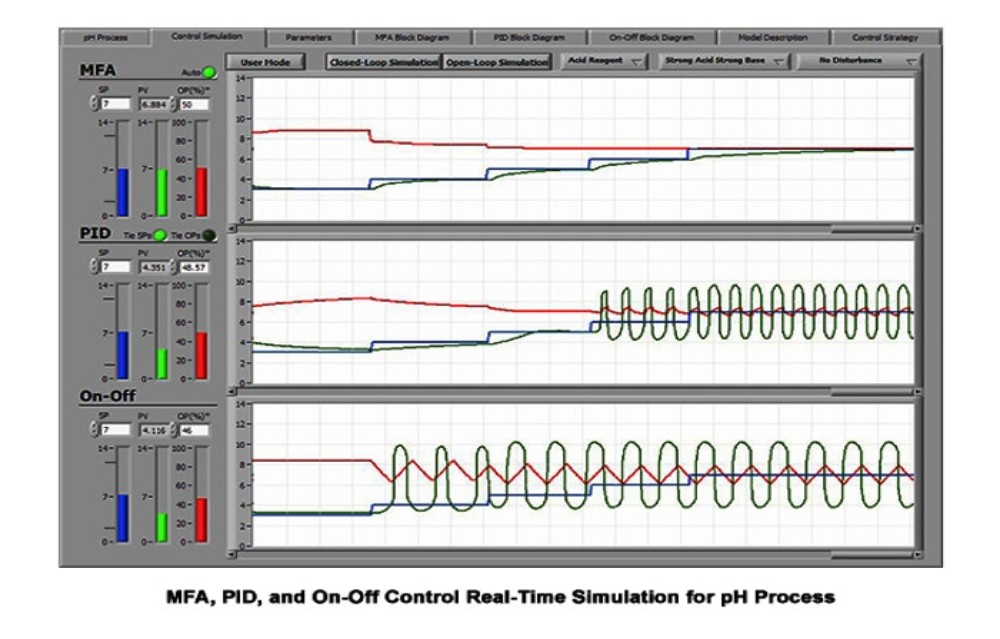
When dealing with acids and bases, the subject of pH and pH control often comes up. In this post we’ll discuss some methods of modelling acidic and basic streams and talk a little about the practical difficulties in controlling pH in the real world.
pH, you’ll hopefully recall, is a logarithmic measure of the activity of hydrogen ions in a solution. For plain water a pH of approximately 7 is “neutral” or natural water, with lower numbers being acidic and higher numbers basic.