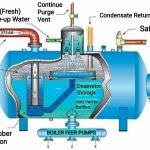
Water is a natural solvent. Rain water is acidic due to carbon dioxide picked up in the atmosphere. Water and CO2 make carbonic acid (acid rain). Water hardness is primarily calcium and magnesium. Calcium is limestone – common throughout Midwest. Acid water dissolves limestone, iron, and other minerals in soil. All pure water has an affinity for metals. Surface water supplies are generally low in dissolved minerals. Well supplies vary in dissolved minerals with depth and location. pH is the measure of acidity of a water supply based on the hydrogen ion. Water with a pH below 7.0 is corrosive. Above is considered alkaline water which is not a problem except in industry.

Posted inGeneral Concepts