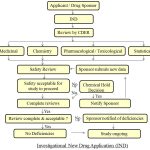
After the IND has not been disapproved within 30 days, then the next phase of testing begins, which is the clinical trials.
Phase IÂ
This phase of the testing takes about a year and involves about 20 to 80 normal, healthy volunteers. The tests study a drug’s safety profile, including the safe dosage range. The studies also determine how a drug is absorbed, distributed, metabolized and excreted, and the duration of its action.Â
Phase IIÂ
In this phase, controlled studies of approximately 100 to 300 volunteer patients (people with the disease) assess the drug’s effectiveness. This phase normally takes about 2 years.
Phase IIIÂ
This phase involves 1,000 to 3,000 patients in clinics and hospitals. Physicians monitor patients closely to determine the efficacy and identify adverse reactions. This phase lasts about three years.Â