There are various methods for the preparation of Ethers. Ethers are the organic compounds containing an oxygen atom bonded to two same or different alkyl or aryl groups. The general formula for ethers can be R-O-R, R-O-Ar or Ar-O-Ar, where R represents alkyl group and Ar represents aryl group. They are generally classified into two categories on the basis of substituent group attached: symmetrical ethers (when two identical groups are attached to the oxygen atom) and asymmetrical ethers (when two different groups are attached to the oxygen atom). With advancements in technologies, ethers are synthesized in industries through many ways. Some of the ways are explained below:
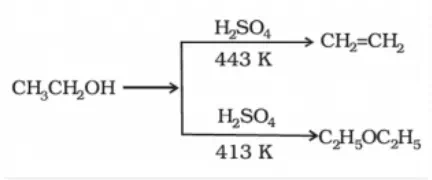
Preparation of ethers by dehydration of alcohols: In the presence of protic acids (sulphuric acid), alcohols undergo dehydration to produce alkenes and ethers under different conditions. For example: in the presence of sulphuric acid, dehydration of ethanol at 443 K yields ethene whereas it yields ethoxyethane at 413 K. This is an ideal method of preparation through primary alcohols.
The preparation of ethers by dehydration of alcohol is a nucleophilic substitution reaction. The alcohol involved in reaction plays two roles: one alcohol molecule act as substrate while the other acts as a nucleophile. It can follow either SN1 or SN2 mechanism. The choice of mechanism depends on whether the protonated alcohol loses water before or simultaneously upon the attack of a second alcohol molecule. Generally, the secondary and tertiary alcohols follow SN1 mechanism while the primary alcohols follow SN2 mechanism.
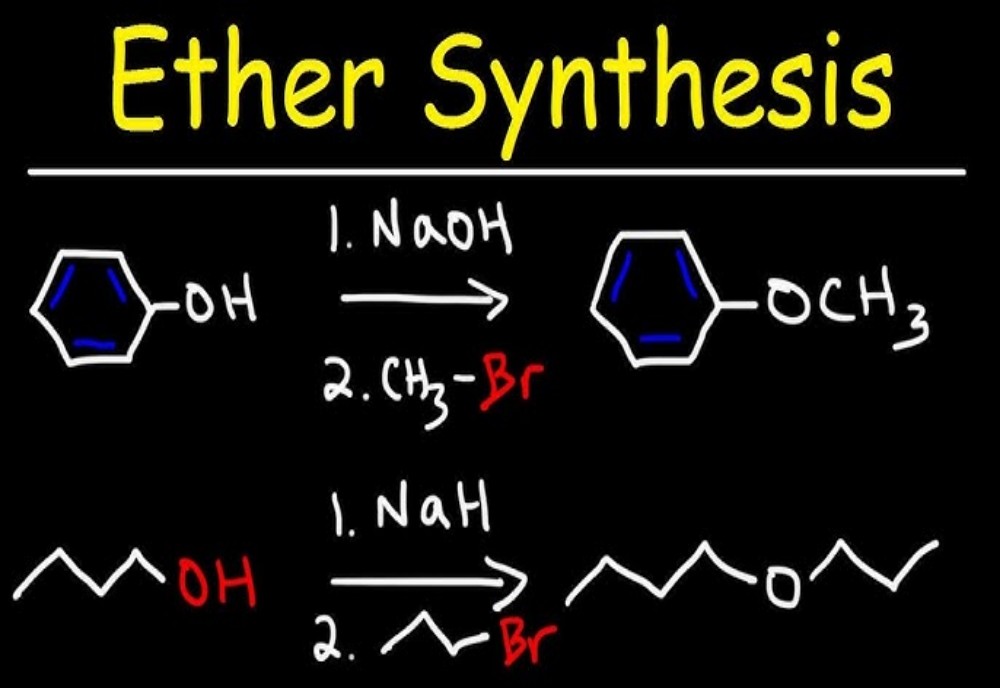
Preparations of ethers by Williamson synthesis: Williamson synthesis is an important method for the preparation of symmetrical and asymmetrical ethers in laboratories. In this method, an alkyl halide is reacted with sodium alkoxide which leads to the formation of ether. The reaction generally follows SN2 mechanism for primary alcohol.

As we know alkoxides are strong bases and they can react with alkyl halides leading to elimination reactions. Williamson synthesis exhibits higher productivity in case of primary alkyl halides. In case of secondary alkyl halides, elimination competes with substitution whereas, we observe the formation of elimination product only in case of tertiary alkyl halides.