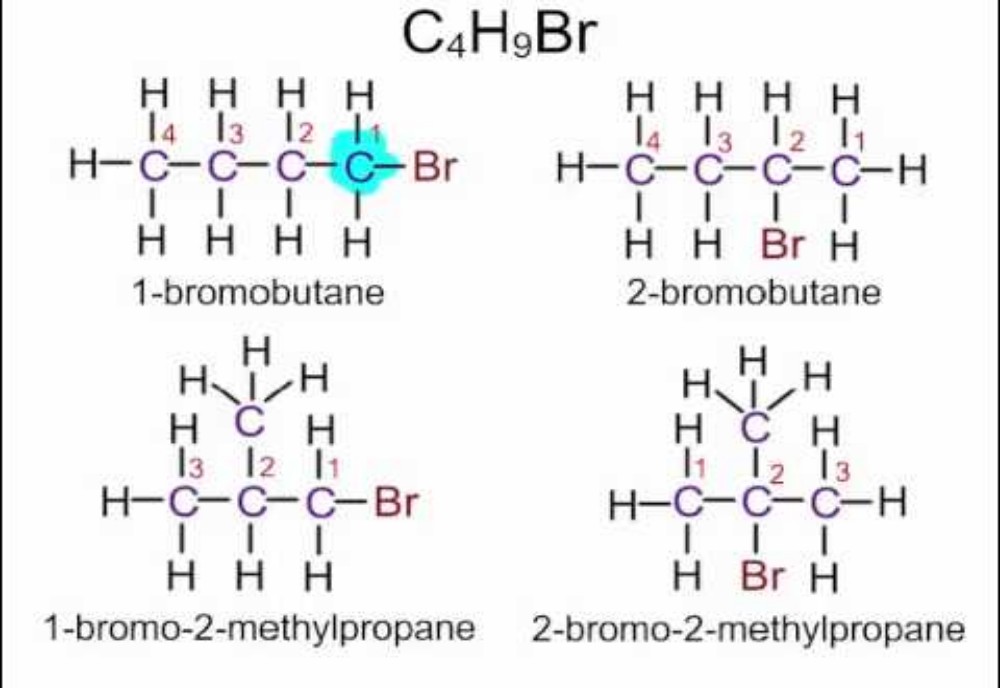
Haloalkanes are hydrocarbons in which hydrogen in normal alkane is replaced by a halogen (group 17 elements). In this article we will discuss about the physical properties of haloalkanes. The physical properties of haloalkanes are mostly like a normal covalent compound. Halogens not being much reactive functional group as carboxyl group or aldehyde doesn’t affect the overall physical properties by much. Still, few differences can be seen as we move down in the homologous series of haloalkanes group due to difference in atomic masses of the compound.
Notable Fact:
Physical properties of any compound depend largely on
Its mass
The type of intermolecular and intramolecular forces of attraction
Melting Point and Boiling Point of haloalkanes
There is large electronegativity difference between halogens and carbon resulting in highly polarized molecules. The higher molecular mass and greater polarity as compared to the parent hydrocarbon results in stronger intermolecular forces of attraction (dipole-dipole and van der Waals) in the halogen derivatives. Boiling Point depends upon the intermolecular forces of attraction and hence the boiling points of chlorides, bromides and iodides are considerably higher than those of the hydrocarbons of comparable molecular mass. As we go down in homologues series of haloalkanes, the forces of attraction becomes stronger due to increase in molecular size and it’s mass, hence the boiling point increases down the homologues series. But the boiling point decreases with branching.
Methyl chloride, methyl bromide, ethyl chloride and some chlorofluoromethanes are gases at room temperature. Higher members are liquids or solids.
Melting point of a compound depends upon the strength of lattice structure of a compound. Melting point also follows the same trend as boiling point. An exception to this is para-isomers. The para-isomers have higher melting as compared to their ortho and meta-isomers. It is due to symmetry of para-isomers that fits in crystal lattice better as compared to ortho– and meta-isomers.
Density of haloalkanes
Density is directly proportional to the mass of compound, hence down the homologous series, density increases, also fluoro derivatives are lesser dense than chloro derivatives; chloro derivatives are less dense than bromo derivatives and so on.
Solubility of haloalkanes
Haloalkanes are slightly soluble in water. This is because of the relatively larger amount of energy required to break bond between halogen and carbon and the smaller amount of energy released, when bond is formed after dissolution ion and water.