Phenols are the organic compounds containing a benzene ring bonded to a hydroxyl group. They are also known as carbolic acids. Thus, a phenol molecule consists of two parts one aryl group part and the other hydroxyl group part.On the basis of number of hydroxyl groups attached to the aryl group, it can be classified into mono-, di-, tri- or polyhydric phenols.
Nomenclature of Phenols:
Earlier, most of the compounds with same structural formula were known by different names depending on the regions where they were synthesised. This naming system was very trivial since it raised a lot of confusion. Finally a common naming system enlisting standard rules was set up by IUPAC for the naming of compounds.
It is both a common name as well as an IUPAC name for the compounds containing a benzene ring attached to a hydroxyl group. Structurally phenols are the simplest hydroxy derivative of benzene ring. IUPAC nomenclature of phenols follows a set of rules.
Rules underlying the nomenclature of phenols:
Locate the position of hydroxyl group attached to the benzene ring.
Benzene rings attached to more than one hydroxyl groups are labeled with the Greek numerical prefixes such as di, tri, tetra to denote the number of similar hydroxyl groups attached to the benzene ring. If two hydroxyl groups are attached to the adjacent carbon atoms of benzene ring, it is named as benzene1,2-diol
In case of substituted phenols, we start locating the positions of the other function groups with respect to the position where the hydroxyl group is attached. For example, if a methyl group is attached at fourth carbon atom with respect to hydroxy group, compound is named as, 4-Methyl phenol.
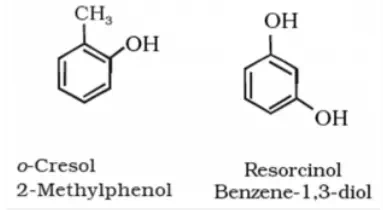
Depending on the position of substituted functional group with respect to the hydroxyl group, words like ortho (when the functional group is attached to the adjacent carbon atom), para (when the functional group is attached to the third carbon atom from the hydroxyl group), meta (when the functional group is attached to the second carbon atom from the hydroxyl group) are also used for the nomenclature of phenols.