S-block elements of the modern periodic table such as lithium and beryllium exhibit unique properties with respect to other elements in the same group due to various reasons.
Lithium: Lithium exhibits distinct properties due to the exceptionally small size of the atom, high polarising power which is a ratio of charge to radius and hydration energy. This increases the covalent nature of lithium compounds.
Anomalous behaviour of lithium with respect to other alkali metals:
• The melting point and boiling point of lithium are higher that than other alkali metals.
• The hardness of lithium is higher than other metals.
• The alkali metal chlorides do not have the capability to form hydrates but lithium chloride crystallizes to form a hydrate LiCl2.H2O
• Lithium nitrate decomposes to form an oxide whereas other metals on heating give nitrites.
• Compounds of lithium are partially soluble in water whereas the alkali metals are highly soluble in water.
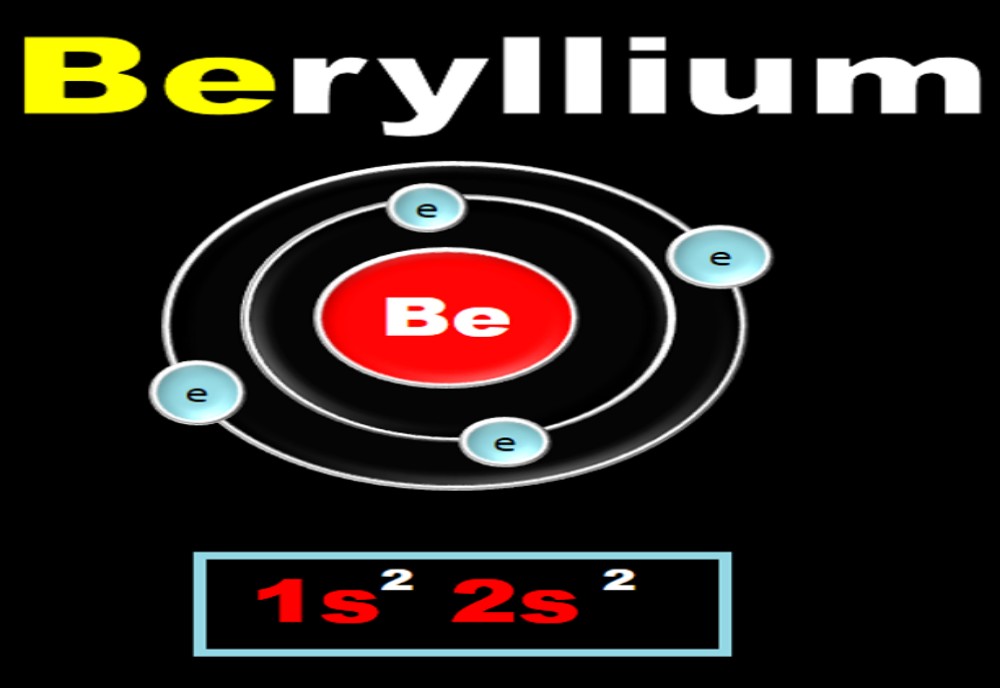
Beryllium: The anomalous properties of beryllium is mainly due to its small size, high electronegativity, high ionization energy and high polarizing power compared to the other elements in the block. The anomalous properties of beryllium compared to other elements of the group are has mentioned below:
• The coordinate number of beryllium is not more than 4 whereas other alkali metals have coordinate number of 6.
• The melting point and boiling points are higher when compared to the other elements of the group.
• They form covalent bonds whereas the other members form of the group form ionic bonds.
• Be does not react with water like the other companions of the group.