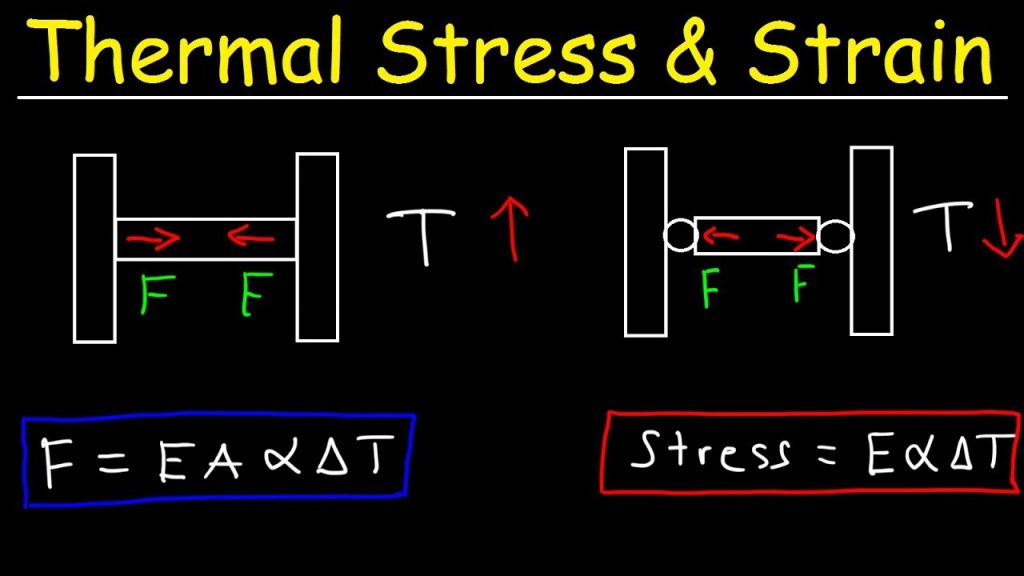
Temperature change can also cause strain. In an isotropic material the thermally induced extensional strains are equal in all directions, and there are no shear strains. In the simplest cases, these thermal strains can be treated as being linear in the temperature change θ − θ0 (where θ0 is the temperature of the reference state), writing εijthermal = δijα(θ − θ0) for the strain produced by temperature change in the absence of stress. Here α is called the coefficient of thermal expansion. Thus, in cases of temperature change, εij is replaced in the stress-strain relations above with εij − εijthermal, with the thermal part given as a function of temperature. Typically, when temperature changes are modest, the small dependence of E and ν on temperature can be neglected.
Anisotropy
Anisotropic solids also are common in nature and technology. Examples are single crystals; polycrystals in which the grains are not completely random in their crystallographic orientation but have a “texture,” typically owing to some plastic or creep flow process that has left a preferred grain orientation; fibrous biological materials such as wood or bone; and composite materials that, on a microscale, either have the structure of reinforcing fibres in a matrix, with fibres oriented in a single direction or in multiple directions (e.g., to ensure strength along more than a single direction), or have the structure of a lamination of thin layers of separate materials. In the most general case, the application of any of the six components of stress induces all six components of strain, and there is no shortage of elastic constants. There would seem to be 6 × 6 = 36 in the most general case, but, as a consequence of the laws of thermodynamics, the maximum number of independent elastic constants is 21 (compared with 2 for isotropic solids). In many cases of practical interest, symmetry considerations reduce the number to far below 21. For example, crystals of cubic symmetry, such as rock salt (NaCl); face-centred cubic metals, such as aluminum, copper, or gold; body-centred cubic metals, such as iron at low temperatures or tungsten; and such nonmetals as diamond, germanium, or silicon have only three independent elastic constants. Solids with a special direction, and with identical properties along any direction perpendicular to that direction, are called transversely isotropic; they have five independent elastic constants. Examples are provided by fibre-reinforced composite materials, with fibres that are randomly emplaced but aligned in a single direction in an isotropic or transversely isotropic matrix, and by single crystals of hexagonal close packing such as zinc.
General linear elastic stress-strain relations have the form
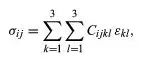
where the coefficients Cijkl are known as the tensor elastic moduli. Because the εkl are symmetric, one may choose Cijkl = Cijlk, and, because the σij are symmetric, Cijkl = Cjikl. Hence the 3 × 3 × 3 × 3 = 81 components of Cijkl reduce to the 6 × 6 = 36 mentioned. In cases of temperature change, the εij above is replaced by εij − εijthermal, where εijthermal = αij(θ − θ0) and αij is the set of thermal strain coefficients, with αij = αji. An alternative matrix notation is sometimes employed, especially in the literature on single crystals. That approach introduces 6-element columns of stress and strain {σ} and {ε}, defined so that the columns, when transposed (superscript T) or laid out as rows, are {σ}T = (σ11, σ22, σ33, σ12, σ23, σ31) and {ε}T = (ε11, ε22, ε33, 2ε12, 2ε23, 2ε31). These forms assure that the scalar {σ}T{dε} ≡ tr([σ][dε]) is an increment of stress working per unit volume. The stress-strain relations are then written {σ} = [c]{ε}, where [c] is the 6 × 6 matrix of elastic moduli. Thus, c13 = C1133, c15 = C1123, c44 = C1212, and so on.