Stoichiometric relations become important, for one!
A->B(1 mole of B made from 1 mole of A)
2B->C(it takes 2 moles of B to make one mole of C)
How many moles of A are necessary to make a mole of C (if no B is fed to the reactor and the reaction goes to completion)
Generation, Consumption terms NOT equal to zero
This makes mass balances more complicated. Need to review/introduce some ideas about reactions.
Stoichiometry
Represents molar participation in a reaction.
How do you do it?
Like a mini mass balance! (Can only balance atomic species NOT total moles or moles (mass) of compounds!)
Left hand side is inlet, right hand side is outlet.
SO2 +02->SO3
Left side 1 S = Right side 1 S (OK so far)
Left side (2 0 + 2 0), Right side 3 0 (NOT BALANCED!)
C4H8 + 6 02 -> 4 CO2 + 4 H20
Left side 4 C = Right side 4 C (OK so far)
Left side 8 H = Right side 8 H (OK so far)
Left side 12 0 = Right side (8 0 + 4 0)(BALANCED!)
So, it takes 6 moles of oxygen (diatomic) to completely combust 1 mole of butane!
Another way to look at it is using a ratio.
6 mole 02 consumed/1 mole C4H8 consumed
OR 4 mole H20 generated/6 Mole 02 consumed
Reactions and Stoichiometry
So, if we had the following reaction:
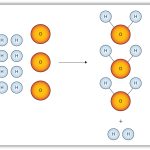
H20 + CO -> CO2 + H2
(is this balanced?)
and we tried to react 28g of CO completely, How many grams of H20 would we need? (Recall that MWCO=28g/mol; MWH20=18 g/mol; MWCO2=44 g/mol; MWH2= 2 g/mol)
How many grams of total products would we get?
What happens if we only have 9g of H20?
DEFINITION
The limiting reactant (reagent) is the one which would run out if we tried to have the reaction proceed to completion. The limiting reactant is present in LESS THAN STOICHIOMETRIC PROPORTIONS!
DEFINITION
The excess reactants (reagents) are the ones which are left over.
How do we quantify the excess?
DEFINITION
The fractional excess is the ratio of the amount by which the feed exceeds stoichiometric requirements (in moles) divided by the stoichiometric requirement (in moles).
It is given (mathematically) by:
XS = (nAreal-nAstoichiometric)/ nAstoichiometric
Where does nAstoichiometric come from? [cheat]