Gases are complicated. They’re full of billions and billions of energetic gas molecules that can collide and possibly interact with each other. Since it’s hard to exactly describe a real gas, people created the concept of an Ideal gas as an approximation that helps us model and predict the behavior of real gases. The term ideal gas refers to a hypothetical gas composed of molecules which follow a few rules:
1. Ideal gas molecules do not attract or repel each other. The only interaction between ideal gas molecules would be an elastic collision upon impact with each other or an elastic collision with the walls of the container.
2. Ideal gas molecules themselves take up no volume. The gas takes up volume since the molecules expand into a large region of space, but the Ideal gas molecules are approximated as point particles that have no volume in and of themselves.
If this sounds too ideal to be true, you’re right. There are no gases that are exactly ideal, but there are plenty of gases that are close enough that the concept of an ideal gas is an extremely useful approximation for many situations. In fact, for temperatures near room temperature and pressures near atmospheric pressure, many of the gases we care about are very nearly ideal.
If the pressure of the gas is too large (e.g. hundreds of times larger than atmospheric pressure), or the temperature is too low (e.g. -200 \text { C}−200 Cminus, 200, start text, space, C, end text) there can be significant deviations from the ideal gas law.
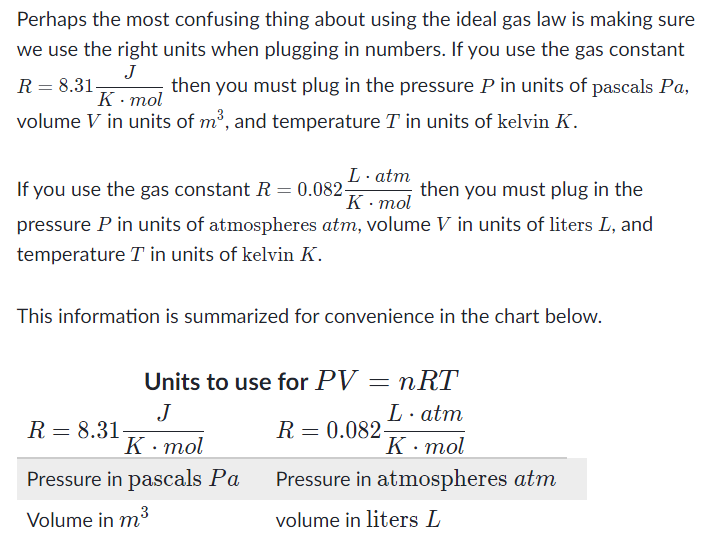
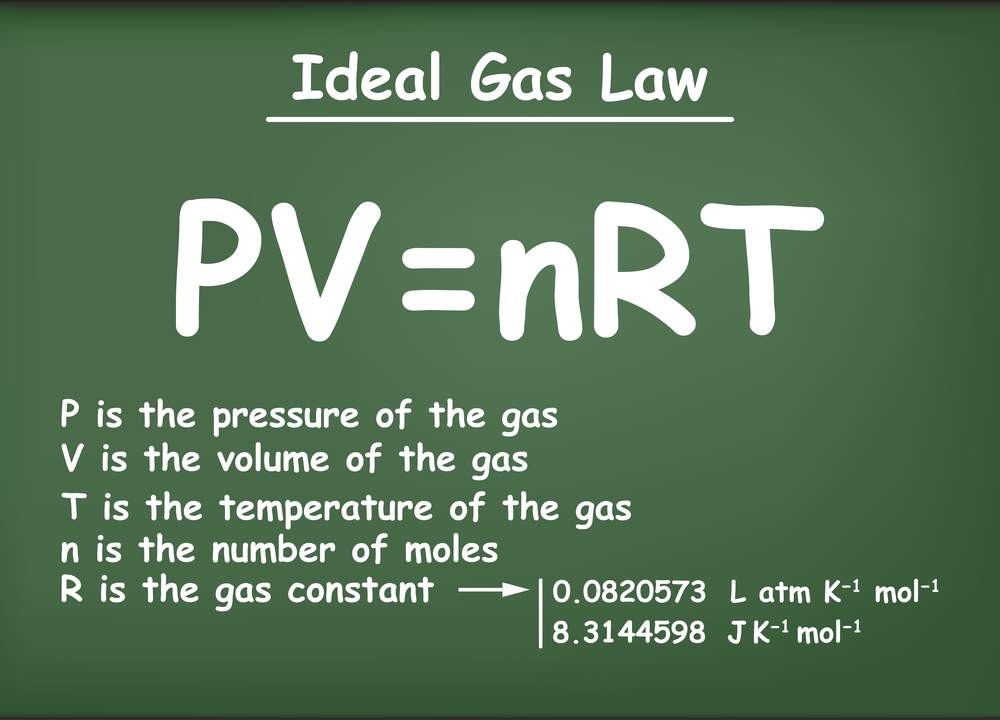
What is the molar form of the ideal gas law?
The pressure, PPP, volume VVV, and temperature TTT of an ideal gas are related by a simple formula called the ideal gas law. The simplicity of this relationship is a big reason why we typically treat gases as ideal, unless there is a good reason to do otherwise.