The force which the substance exerts on another substance per unit area is known as pressure. The pressure of the gas is the force that the gas exerts on the container boundaries. The gas molecules move randomly along the given volume. During this movement, they collide with the surface and also with each other. The impact of every individual gas molecule is too small and difficult to visualize. But the impact of all the gas molecules considered together constitutes the gas pressure. Greater the number of collisions, greater would be the pressure.
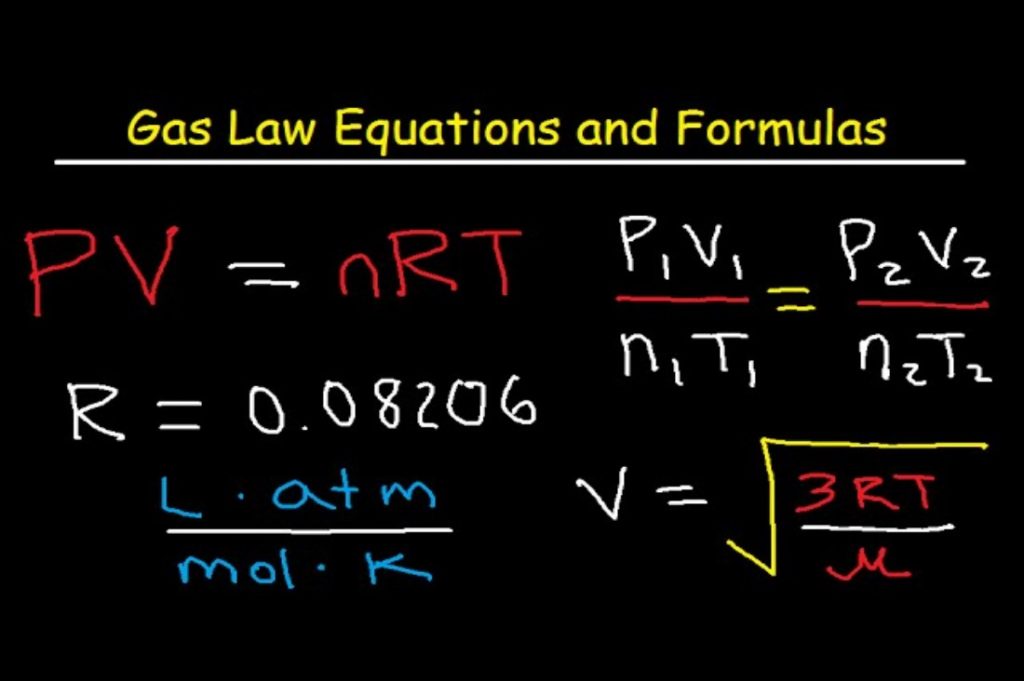
The Gas pressure formula is given as,
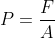
Where,
F = impact force due to gas collisions in Newtons (N),
A = area in meter square
The Ideal gas pressure formula is given as,
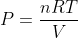
Where,
V = volume,
n = number of moles,
R = Gas constant, (8.3145 Jol/mol/K)
T = temperature.
The SI unit for Gas pressure is expressed in Pascals (Pa).
Example 1
There are 500 moles of gas molecules in a container. If 220 K temperature is applied to the gas of a volume of 40 L, identify the Gas pressure.
Solution
Given :
No of moles n = 500 mol,
temperature T = 220 K,
Volume V = 40 L
The Gas pressure formula is given by,
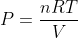
P = 500 ×0.082×220 / 40
P = 225.5 Pascal
Example 2
If the Gas molecules move with the force of 300 N in the area of 50 m2. Determine its gas pressure.
Solution:
Given:
Force F = 300 N,
area A = 50 m2
The gas pressure formula is given by,
P = F / A
P = 300 / 50
P = 6 Pascal