Case (a): The liquid component B is not significantly broken at the interface if the concentration of B is large in comparison with cA, then it reduces to a pseudofirst-order reaction.
Case (b): When components A and B react so quickly that they cannot coexist at the same location to any significant extent (‘instantaneous reaction’). The film model gives for this case

where ‘ d is the reaction plane at the condition vBjA = -jB in which the concentration of both components is equal to zero. It is a function of the diffusion rate of A and B and of the whole boundary thickness which can be expressed as:

Substituting the Equation (4.65) in Equation (4.64), the absorption rate can be written as:
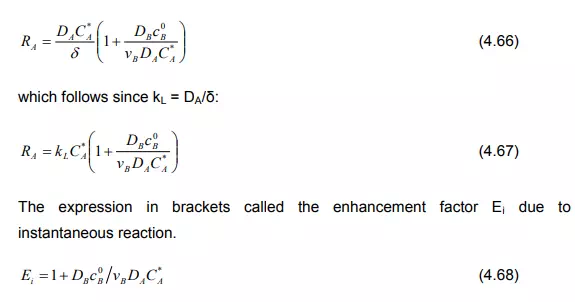

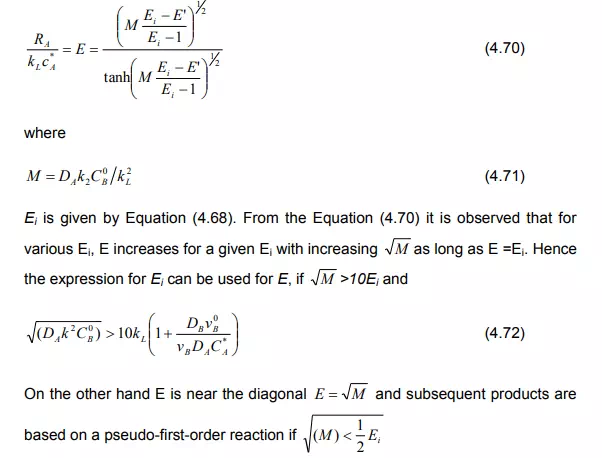
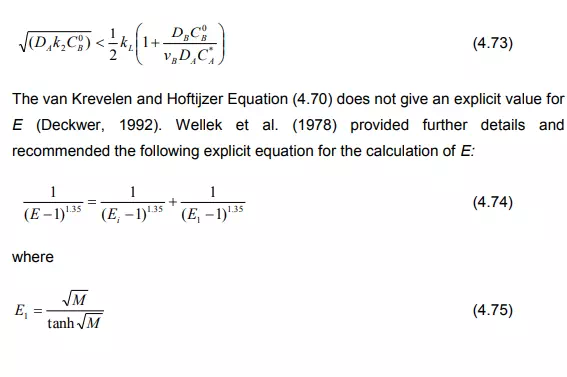
Case (c): If the concentration of B drops distinctly in comparison with the bulk concentration, yet does not reach zero, the film model produces two coupled differential equations which can be solved numerically. Van Krevelen and Hoftijzer (1948) have provided an approximated solution as: