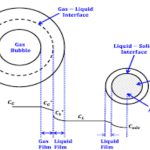
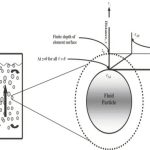
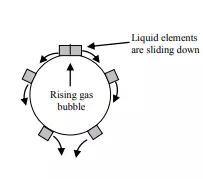
Most of the industrial processes of mass transfer is unsteady state process. In such cases, the contact time between phases is too short to achieve a stationary state. This non stationary phenomenon is not generally taken into account by the film model. In the absorption of gases from bubbles or absorption by wetted-wall columns, the mass transfer surface is formed instantaneously and transient diffusion of the material takes place. Figure 3.4 demonstrates the schematic of penetration model. Basic assumptions of the penetration theory are as follows:
1) Unsteady state mass transfer occurs to a liquid element so long it is in contact with the bubbles or other phase
2) Equilibrium exists at gas-liquid interface
3) Each of liquid elements stays in contact with the gas for same period of time
Under these circumstances, the convective terms in the diffusion can be neglected and the unsteady state mass transfer of gas (penetration) to the liquid element can be written as:
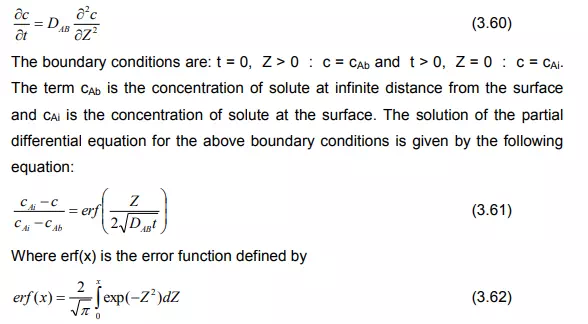
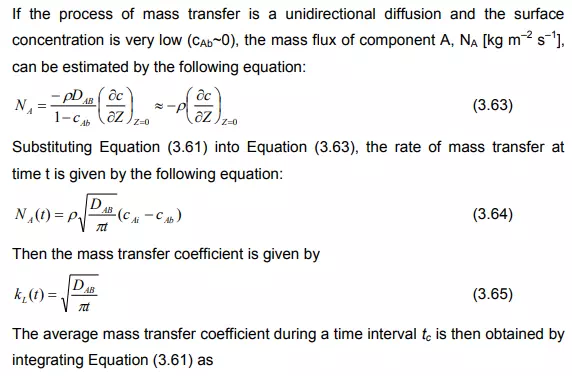

So from the above equation, the mass transfer coefficient is proportional to the square root of the diffusivity. This was first proposed by R. Higbie in 1935 and the theory is called Higbie’s penetration theory.