Heat transfer is the process of transfer of heat from high temperature reservoir to low temperature reservoir. In terms of the thermodynamic system, heat transfer is the movement of heat across the boundary of the system due to temperature difference between the system and the surroundings. The heat transfer can also take place within the system due to temperature difference at various points inside the system. The difference in temperature is considered to be ‘potential’ that causes the flow of heat and the heat itself is called as flux.
Modes of Heat Transfer
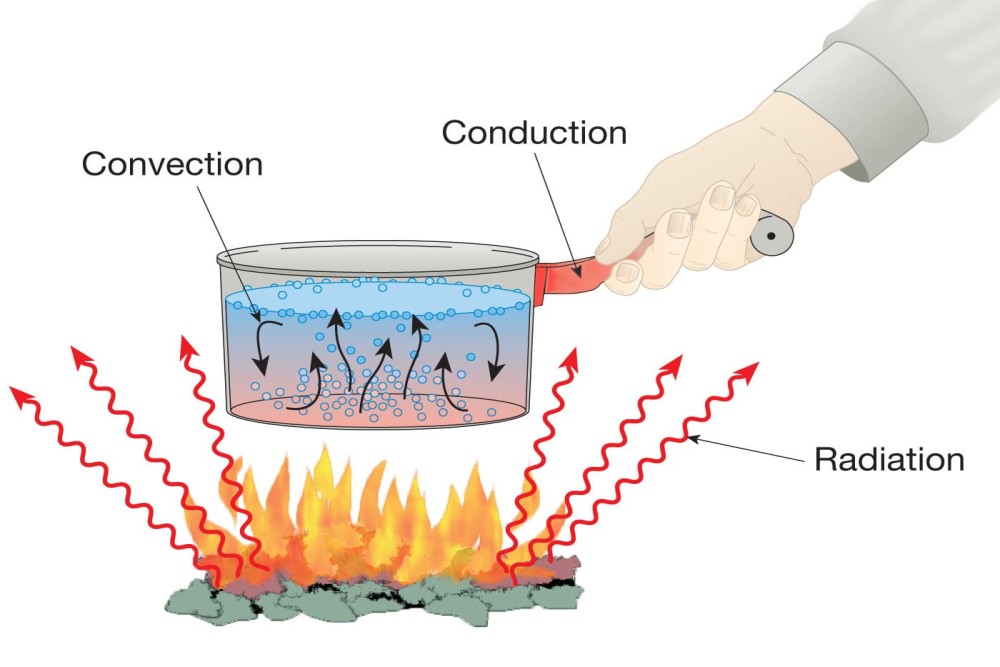
There are three modes of heat transfer between the two bodies: conduction, convection and radiation. These have been described below:
Conduction: The transfer of heat between two solid bodies is called as conduction. It depends on the difference in temperature of the hot and cold body. Example of conduction heat transfer is two bodies at different temperature kept in contact with each other. Another example is heating one end of the metal like copper; due to conduction heat transfer the other end of the metal also gets heated.
Convection: The transfer of heat between the solid surface and the liquid is called as convection heat transfer. Let us considering a vessel of water being heated, in this case heating of water due to transfer of heat from the vessel is convection heat transfer.
Radiation: When two bodies are at different temperatures and separated by distance, the heat transfer between them is called as radiation heat transfer. In case of the conduction and convection heat transfer there is a media to transfer the heat, but in case of the radiation heat transfer there is no media. The radiation heat transfer occurs due to the electromagnetic waves that exist in the atmosphere. One of the most important examples of radiation heat transfer is the heat of the sun coming on the earth.
Heat Transfer as Per Second Law of Thermodynamics
As per the second law of thermodynamics the transfer of heat takes place from the body of high temperature to the body of low temperature. There won’t be spontaneous transfer of heat from the body at low temperature to the body at high temperature. For heat transfer from low temperature body to high temperature body, external work has to be done.
The heat gained by the system or body is considered to be positive and the heat lost by the system is considered to be negative for the mathematical calculations. This implies that the heat flowing into the system is positive and heat flowing out of the system is negative. The amount of heat transfer is denoted by symbol Q.


Comments are closed