Calcination treatments in the range of 500–900 °C of TiO2 synthesised by the sol–gel resulted in materials with variable physicochemical (i.e., optical, specific surface area, crystallite size and crystalline phase) and morphological properties. The photocatalytic performance of the prepared materials was evaluated in the oxygen evolution reaction (OER) following UV-LED irradiation of aqueous solutions containing iron ions as sacrificial electron acceptors. The highest activity for water oxidation was obtained with the photocatalyst thermally treated at 700 °C (TiO2-700). Photocatalysts with larger anatase to rutile ratio of the crystalline phases and higher surface density of oxygen vacancies (defects) displayed the best performance in OER. The oxygen defects at the photocatalyst surface have proven to be responsible for the enhanced photoactivity, acting as important active adsorption sites for water oxidation. Seeking technological application, water oxidation was accomplished by immobilising the photocatalyst with the highest OER rate measured under the established batch conditions (TiO2-700). Experiments operating under continuous mode revealed a remarkable efficiency for oxygen production, exceeding 12% of the apparent quantum efficiency (AQE) at 384 nm (UV-LED system) compared to the batch operation mode.
Introduction
Oxygen is used in numerous industrial processes, for example, synthesis of chemicals, petroleum processing, glass, ceramic, pulp and paper manufacture. It is mainly used to enhance the reaction rates and to ensure the complete oxidation of possible undesired by-products occurring during some processes1. Unfortunately, the transport and delivery of gaseous molecular oxygen pose several treads to its industrial application. Production in situ is undoubtedly an alternative forwarded by many technological strategies.
Therefore, the demand for oxygen production has encouraged the scientific community to expand the concept of artificial photosynthesis that mimics nature to convert solar energy into value-added products. Since the pioneering work of Fujishima and Honda in 19722, reporting the development of a photoelectrochemical cell for the decomposition of water into hydrogen and oxygen under UV light irradiation using the optical semiconductor titanium dioxide (TiO2), research in this field became an important topic both for environmental decontamination and energy conversion. A variety of optical semiconductors (e.g., TiO2, zinc oxide (ZnO), tungsten trioxide (WO3), graphitic carbon nitride (g-C3N4), bismuth-based materials, black phosphorus, and others) have been employed in several photoactivated applications, including degradation of domestic and industrial sewage, and conversion of natural resources, such as carbon dioxide, nitrogen, and water to value-added chemical and fuels.
Concerning water splitting using semiconductor photocatalysis, the process consists of two half-catalytic redox reactions, i.e., the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER), which are described by the following Eqs. (1) and (2), respectively:2H++2e−→H22H++2e−→H2(1)2H2O+4h+→O2+4H+2H2O+4h+→O2+4H+(2)
The OER is viewed as a bottleneck for the energy conversion due to its slow kinetics11, which involves four proton-coupled electron transfer steps. These elementary stages (i.e., charge carrier generation, separation and migration) occur during the photocatalytic water oxidation, followed by the surface catalytic reaction. Many efforts have been pursued on developing photocatalysts to accelerate the water oxidation, such as junction construction of metal oxides and by metal deposition, aiming to decrease the energy barrier10,12. Despite the significant advances in photocatalysts development neat TiO2 continues to receive particular attention due to the nature of the physical interactions between anatase and rutile crystalline phases, which may result in unique trap states and therefore can have a significant impact on the interparticle carrier transport13.
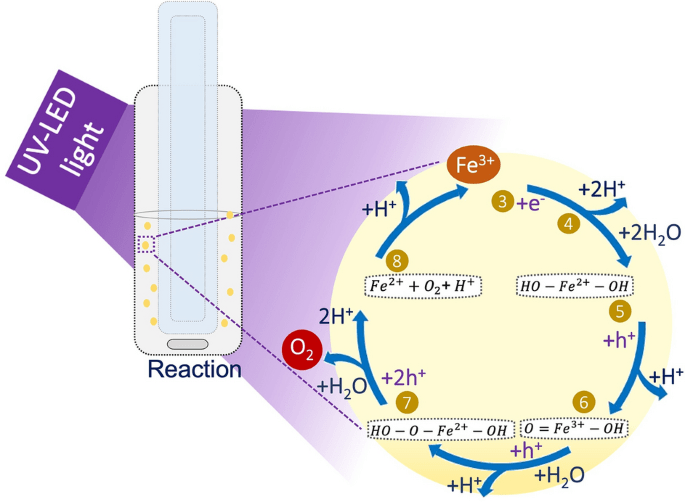
Although the catalyst nature is of high importance concerning the water splitting systems, the major limitation is its relatively low efficiency in the presence of pure water, requiring the addition of sacrificial agents. Thus, many examples exist of sacrificial molecules as electron donors to improve hydrogen production in solar-driven water splitting systems14,15,16. However, only a limited variety of electron acceptors have been considered concerning OER. The frequently used electron acceptors are silver cations (Ag+), yet some problems arise from their use, i.e., generally, the photocatalytic formation of molecular oxygen is accompanied by the deposition of metallic silver (Ag0) nanoparticles, causing irreversible optical changes on the semiconductor properties. Slightly, ferric ions (Fe3+) have been employed as sacrificial electron acceptors to produce oxygen17,18 owing to its lower redox potential in water (+ 0.77 V; vs. NHE) over Ag+ (+ 0.80 V; vs. NHE), and due to the reversible redox couple Fe3+/Fe2+ in the aqueous phase compared with the Ag+ ions. The influence of Fe3+ ions as electron acceptors was documented by Matsumura group17. In this study, the authors used a TiO2 rutile material for water oxidation and found that the reaction is favoured by superior adsorption of Fe3+ ions on TiO2 over the Fe2+ ions.
Therefore, due to the crucial importance of O2 in various processes this work explores in situ oxygen production by water oxidation based on semiconductor photocatalysis technology. The influence of oxygen defects, optical and crystalline properties of TiO2 samples calcined at different temperatures was investigated for oxygen evolution using a low concentration of Fe3+ aqueous solution rather than the typical electron acceptor (Ag+). The lower price, high abundance and broad availability of iron compared to silver, the use of Fe3+/Fe2+ in low concentrations, makes this a cost-effective and sustainable process. In addition, considering the advantages of using continuous mode systems, a selected TiO2 sample was deposited in a glass support, and the rate of oxygen production was assessed. To the best of our knowledge, this is the first study on photocatalytic water oxidation using immobilised TiO2 towards in situ oxygen production under continuous mode, avoiding the need of recovery the catalyst as an additional step.
Results and discussion
X-ray diffraction (XRD) analysis was performed to determine the crystalline phase and crystallite size of TiO2 samples The peaks appearing at 25° and 48°, correspond to the lattice planes of 101 and 200 (JCPDS 21-1272) of TiO2 anatase phase. The peaks at 27°, 36° and 54° with lattice planes of 110,101 and 211 (JCPDS 21-1276), respectively reveal the presence of rutile phase. the diffraction peaks of both anatase and rutile phases of TiO2 samples become intense as the calcination temperature increases, suggesting that TiO2 samples are composed of irregular polycrystalline materials, and the crystallinity for both phases is improved by rising the temperatures.