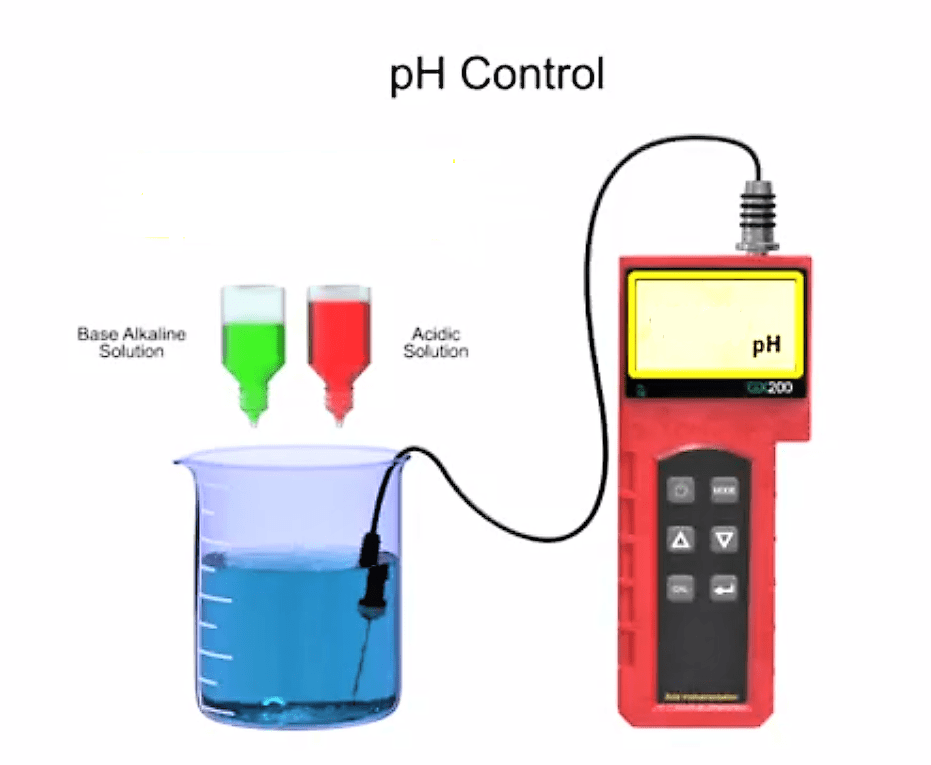
pH can be surprisingly difficult to control. It seems simple: why not just install a pH probe and have an injection pump add acids and/or bases into your process stream?
One reason pH control is so difficult is the logarithmic nature of the pH measurement. The logarithms amplify changes near the neutral point of your solution: every step further from the neutral point will be “10 times bigger” than the step before it.
For example, if you have a strong acid AND a strong base in water, pHs near 7.0 are very difficult to target: small nudges in concentration can very easily send you to pHs of 6.0 or 8.0. However, as the pH gets very high (towards 14) or low (towards 1) it takes much larger changes to affect the pH of the system, literally many 1000’s of times larger.
If you see a wide range of pH values this can create a major challenge for designing and tuning your controls: your system behavior and sensitivity can vary tremendously depending on what pH you are at. It is very hard to get a pump and control valve and control programming that can handle the extremes and the middle. The result is that if you need to take a system from a very high or low pH toward neutrality, you may need to use several steps, and multiple mixing tanks, to achieve your results. E.g. one mixing system for pH 1 to 5, and another from 5 to 7. And because of the sensitivity involved,mixing your fluid well and placing your pH probe in the correct spot downstream of your mixer is crucial.