Consider a closed system comprised of two, first-order, irreversible (one-way) elementary reactions with rate constants kl and k2 :
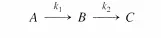
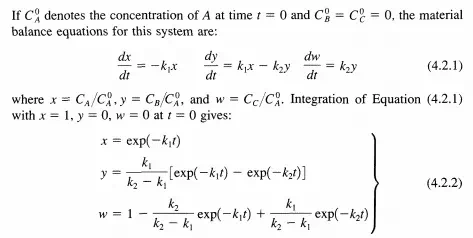

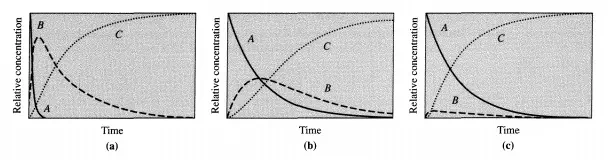
This is the analytical expression of the steady-state approximation: the time d erivatives of the concentrations of reactive intermediates are equal to zero. Equation (4.2.9) must not be integrated since the result that y = constant is false [see Equation (4.2.7)]. What is important is that B varies with time implicitly through A and thus with the changes in A (a stable reactant).


