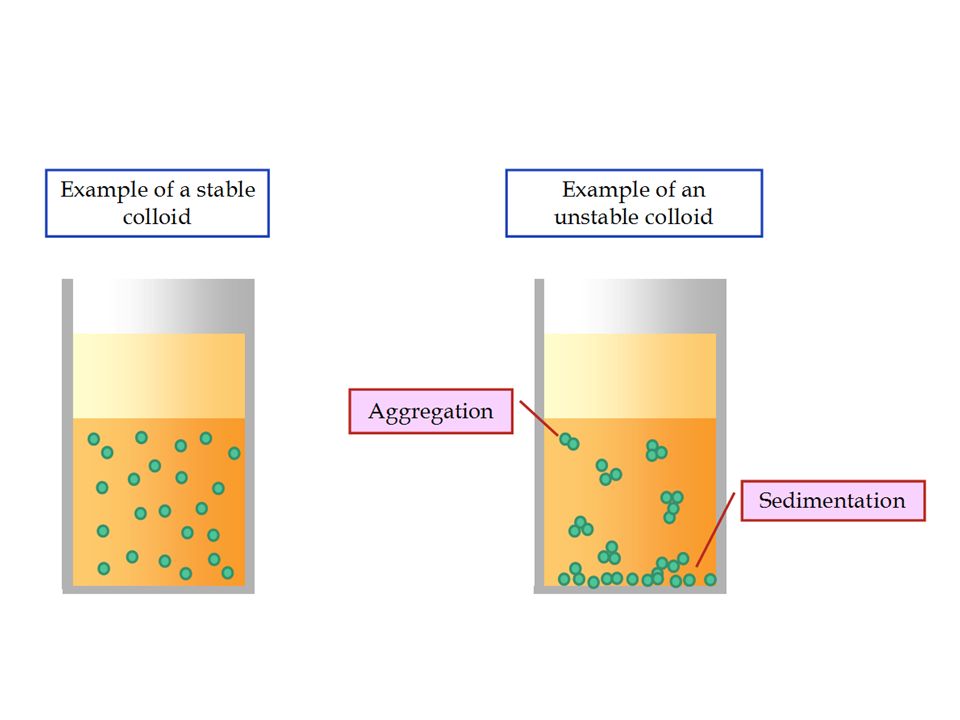
Colloids can be prepared by a variety of techniques involving physical, chemical as well as some dispersion methods. These techniques are based on a variety of principles. Let us explore more these techniques for Preparation of Colloids.
Preparation of Colloids
However, there are two principal ways of preparation of colloids:
· Dispersion of large particles or droplets to the colloidal dimensions by milling, spraying, or application of shear (e.g. shaking, mixing, or high shear mixing).
· Condensation of small dissolved molecules into larger colloidal particles by precipitation, condensation, or redox reactions. Such processes are used in the preparation of colloidal silica or gold.
Chemical Methods of Preparation of Colloids
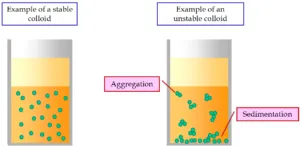
Hydrophilic or Lyophobic colloidal solutions can be prepared by various chemical techniques such as:
· Double Decomposition Technique: When hydrogen sulphide is passed through a solution of arsenious oxide in distilled water, we get a colloidal solution of arsenious chloride.
As2O3 + 3H2S → As2S3 + 3H2O
· Oxidation Technique: A colloidal solution of Sulphur is made to pass through an aqueous solution of sulphur dioxide. It can also be obtained by passing the gas through a solution of an oxidization agent such as bromine water as well as nitric acid.
SO2 + 2H2S → 2H2O + 3S
H2S + [O] → H2O + S
· Reduction Technique: Another technique of preparing colloidal solutions of metals such as silver, gold as well as platinum involves the use of reducing agent for reduction of the salt solutions of these metals. Example of reducing agent include stannous chloride.
· Hydrolysis Technique: It involves the use of boiling water to obtain a reduced solution of ferric chloride.
FeCl3 + 3H2O → Fe(OH)3 + 3 HCl
Physical Methods of Preparation
Various physical methods can also be employed to obtain colloidal dispersions. Some of them include
Exchange of Solvent
It involves the formation of a colloidal solution of an element by addition of its alcoholic solution to excess water. This colloidal formation can take place only when the element is more soluble in alcohol as compared to water. Example: When an alcoholic solution of sulphur is made to pass through excess water it yields a colloidal solution of sulfur. This is because the solubility of sulphur is more in water as compared to alcohol.
The Technique Involving Excessive Cooling
This technique involves freezing a solution of water in organic solvents such as chloroform, ether, etc to form colloidal solutions of ice. The molecules of water find it impossible to exist separately combine to form colloidal molecules.
Dispersion Techniques
These involve electrical disintegration and peptidization.
· Electrical Disintegration: It is the combination of dispersion and condensation. This technique is most commonly used for the preparation of colloidal solutions of metals such as gold, silver, platinum, etc. It involves the use of two metal electrodes dipped in a dispersion medium. An electrical arc of intense heat is produced vaporizing some of the metal. The vapours condense to form precipitates which are of colloidal dimensions.
· Peptidization: Precipitate can be converted into colloidal form by shaking with dispersion medium in the presence of small amount of peptidization agent. It is an electrolyte used to convert fresh precipitate into a colloidal solution. Such precipitates are aggregates of colloidal particles held by weak forces.
Solved Questions For You
Que: Which of the following is NOT a technique for preparation of colloids?
a. Double Decomposition
b. Dispersion technique
c. Excessive cooling Technique
d. Vulcanization
Ans: The correct answer is option “D”. Vulcanization is not a technique for the preparation of colloids. Rest other methods are used in the preparation of colloids.