Abstract
Biocatalysis is a growing area of synthetic and process chemistry with the ability to deliver not only improved processes for the synthesis of existing compounds, but also new routes to new compounds. In order to assess the many options and strategies available to an engineer developing a new biocatalytic process, it is essential to carry out a systematic evaluation to progress rapidly and ensure decisions are made on firm foundations. In this way, directed development can be carried out and the chances of implementation of a commercially successful process can be much improved. In this review we outline the benefits of reaction engineering in this development process, with particular emphasis of reaction kinetics. Future research needs to focus on rapid methods to collect such data at sufficient accuracy that it can be used for the effective design of new biocatalytic processes.
Introduction
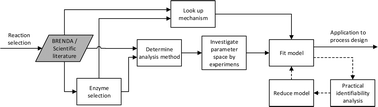
In recent decades a growing branch of synthetic chemistry has been established which uses enzymes to catalyze interesting reactions for the production of valuable molecules. Such an approach is termed biocatalysis and today finds application in the synthesis of many chemical products, ranging from bulk commodities to pharmaceutical intermediates. Several hundred industrial processes have already been implemented, mostly in the pharmaceutical industry, with more in development. The motivation for the application of such catalysts stems from their ability to perform highly selective chemistry under mild conditions in water based solutions, making them attractive as ‘green’ catalysts.8 In the last decade the ability to alter the properties of the enzyme via protein engineering has enabled the synthesis of entirely new molecules and reactions (without precedent in nature). Multi-step sequences of enzymes, operating sequentially or in tandem,13,14 as well as chemo-enzymatic combinations15,16 have now also been established. In short, biocatalysis provides a valuable tool to complement many established synthetic approaches. Despite these scientific developments biocatalysis is still often limited in application due to a poor transition from the laboratory to the process plant. There are several good reasons for this, but amongst the most important is the complexity of enzyme kinetics, combined with the fact that the enzymes need to carry out synthetic reactions under conditions far away from those found in Nature. This makes the collection of parameters in kinetic models especially difficult. For conventional chemical reactions (including catalytic conversions), reaction engineering has long provided an efficient and effective methodology for the design and sizing of appropriate reactors in which to synthesize valuable industrial chemicals.17–20 At the heart of the discipline lies the determination of rate laws, collection of kinetic parameters and the application of these models to mass balances to enable in silicoprediction of product concentration and reactant conversion as a function of residence time. It is an essential activity to inform chemical engineers charged with the design of pilot-scale or full scale plant. The time is now right for the development of such a paradigm for biocatalytic reactions where suitable methods are established for deriving kinetic expressions, not solely aimed at the mechanistic understanding required by biochemists, but now also of appropriate accuracy to be used by (bio)chemical engineers to design (bio)reactors. Such design should also include options for defining suitable biocatalyst loadings and operating schemes to make optimal use of existing equipment, which is often the requirement in the pharmaceutical industry. Likewise such design should also enable considerations for improvement of the enzyme itself21 (via protein engineering) as well as the process plant and operation to be considered.
In order for the new biocatalytic synthesis routes to reach industrialization it is necessary to have models describing the kinetic properties of the biocatalyst. Chemical engineering tools can then be used to scale and design facilities. Ideally, for the biocatalyst to reach this stage several requirements need to be met.
• An enzyme has been developed to thrive in the operational conditions required in the industrial process, frequently much harsher than those found in Nature. For example, a process for pharmaceutical synthesis, requires product concentrations of >50 g L−1,3,6,22,23 with a biocatalyst yield of 10–100 gProduct gImmobilized Biocatalyst−1.22,23
• The enzyme has been characterized comprehensively in terms of kinetics and stability.
• A model has been fitted to describe the rate of reaction in the full conversion range.
• A process concept has been made to define targets for the performance of the enzyme.
These four requirements are often attained in an iterative manner, leading to inefficiencies. Systematic procedures would be far more preferable to give the opportunity to assess the feasibility of processes quickly and where appropriate design optimum development strategies. Enzyme kinetics lies at the center of this procedure.
Today processes are developed first with an emphasis on protein engineering to broaden substrate scope, and secondly by process engineering to enable implementation. However, it is our contention that investigation of potential processes should be considered much earlier in the development procedure, so that it is possible to use reaction engineering as a guide for protein engineering such that biocatalytic properties match the process requirements. Indeed, without such guidance there is even a danger of ‘over’-engineering an enzyme. We believe judicious use of process engineering in concert with protein engineering may ultimately prove more effective.
With this background to the importance of kinetics, we will in this review describe different kinetic models of enzymes important to synthesis and production, and describe methods available for determination of rate laws (and associated kinetic parameters). Importantly, we will describe the application of such models in process evaluation and design and give a future outlook, emphasizing where they can be used to assist the targeted improvement of the biocatalysts themselves.
Biocatalytic process features
As described in numerous texts, chemical reaction engineering is built around the determination of a rate law (defining the relationship of the rate of reaction with the concentration of reactants and catalyst, under given conditions). Although in essence the rate law is similar whether an enzymatic or a chemical catalyst is used (e.g.Michaelis–Menten kinetics are equivalent to Langmuir–Hinshelwood), in reality extra terms are required in enzyme catalysis to account for reactant and product inhibition at the extraordinarily high concentrations required for an industrial process, compared to those found in Nature. This added complexity needs to be built into the rate law and becomes particularly important when multiple reactants are used and/or products produced. Hence the rate law may prove particularly complex and while the estimation of macro-kinetic parameters is difficult, the estimation of micro-kinetic parameters is in many cases impossible due to problems of identifiability.
A second feature of enzyme reactions is that they usually take place in the liquid phase. This means that operating a simple continuous plug flow reactor for catalyst characterization, is frequently limited due to high pressure drops. The many chemical reactions that take place in a gas phase can easily overcome such problems, due to much lower viscosities and higher diffusion rates. Additionally, enzyme reactions in Nature mostly take place in an aqueous environment, and while many enzymes have the ability to work in organic media (to a greater or lesser extent), clearly the kinetics are affected. In many cases the requirement for addition of an organic solvent is essential based on the poor water-solubility of many of the most interesting industrial compounds. The complex structure of an enzyme also means that the protein is subject to unfolding under exposure to extremes of pH, temperature, ionic strength and interfacial effects. In general, conditions such as the solvent, pH and temperature will therefore be predefined, but in principle this also provides room for optimization, provided suitable kinetic data is available as a function of these variables. In itself this also implies a vast space of reaction conditions.
The third important feature of biocatalysis, with respect to reaction engineering, concerns thermodynamics. The early days of biocatalysis focused in particular on hydrolytic reactions, in the presence of water. Since then we now know that the amount of water required to maintain structure is minimal (although essential) meaning such reactions can be run in reverse.26,27 Biocatalysts lowers the activation energy for both directions of a reaction and thermodynamics determines the favourable direction. Nonetheless, it can be desirable to operate reactions in the unfavourable direction for synthetic purposes. Specific products, low cost substrates or natural substrates can be the motivator for such a direction of reaction, but makes the process considerable more difficult to design. Substrate and consequently product pairs can however be chosen so that the direction of the reaction will be overall favourable. This has been shown for amine transaminases and can also be obtained by coupling the main reaction with enzymatic cascades. In cases where reactions are operated against the thermodynamically favourable direction it is necessary to collect thermodynamic data to establish the reaction equilibrium as well as the kinetic data. Unlike chemical catalysts where the variables of pressure and/or temperature can be used to shift equilibrium, for biocatalysis other methods are required such as use of an excess of a reactant (provided it is beneath its inhibitory threshold) and in situproduct removal (ISPR technologies). This also needs consideration in reaction engineering.
For all these reasons we argue that biocatalysis is deserving of a separate treatment in reaction engineering. The variables available to improve the process metrics, as well as the targets required, are quite different depending on whether one develops a chemo-catalytic reaction or a biocatalytic reaction. For example, the operational temperature for chemo-catalysts can span hundreds of degrees and investigation of rate constants can be extrapolated by activation energies to describe this change. The different activation energies of parallel reactions can then be used to tune selectivity. In contrast, the temperature range for enzymes is rather limited and selectivity rarely a concern.

Comments are closed