There has been quite a number of aerobic oxidation reactions reported in the organic process development literature in recent years. Case studies selected from the fine chemicals/pharmaceutical industry are presented below. These examples include uncatalyzed reactions, as well as those enabled by homogeneous and heterogeneous catalysts. In some cases, multi-kilograms of products can be obtained, although commercial production using these processes has yet to be realised. Most of these processes are performed initially in batch reactors, although continuous flow systems have also been described in some cases.
It is often more practical to adopt heterogeneous catalysis in reaction scale up, as it is generally more amenable to process intensification (e.g. continuous flow), and the catalyst can be easily separated from the reaction mixture. However, in a recent review of heterogeneously-catalysed alcohol oxidation for the fine chemical industry,77 none of the cited work has translated into commercial processes. A rare example of a heterogeneously-catalysed aerobic oxidation reaction can be found in a process developed by GlaxoSmithKline (GSK) for the synthesis of an hepatitis C virus (HCV) replicase inhibitor.78 The synthetic route requires the double oxidation of a diol (13) into a dialdehyde (14), which condenses with a protected hydrazine to produce a 5H-imidazo[4,5-d]pyridazine (15) (Scheme 9). In the early stages of the program, the double oxidation was carried out on a 600 g scale using an excess of MnO2 as the oxidant, requiring 5.7 kg of the oxidant to reach completion, giving a 69% yield of 15. A screen for heterogeneous catalysts capable of effecting the aerobic oxidation of diol 13 was subsequently performed, including 106 combinations of catalysts (Ru, Pt, Pd, and Bi additives), loading, solvent and basic additives. This led to the identification of 5% Pt/Bi on C in acetonitrile with added potassium hydroxide as the best performer. The reaction was carried out on 2 g scale by vigorously stirring the reaction mixture in open air at 45 °C; the dialdehyde (14) formed contained some impurities but was telescoped through a hydrazine condensation process, forming the desired product 15in 70% overall yield. Unfortunately, the cancellation of this project stopped further investigation into the oxidation; however, the catalytic process is unquestionably more atom-economical and has greater potential for scale-up.
Scheme 9 GSK route to the aerobic oxidation of a diol (13) to a dialdehyde intermediate (14).
6-Hydroxybuspirone (16) is a metabolite of the anxiolytic drug buspirone (17) which shows good affinity for the same 5-HT1A receptor as the parent drug.79 Challenged with the production of 100 kg quantities of 16 to support toxicological and clinical studies, Bristol-Myers Squibb developed a route based on the Gardner modification80of a Barton enolate oxidation81 whereby the enolate of buspirone is treated with O2 to generate a hydroperoxide (18), which is reduced in situ by a phosphite to 16 (Scheme 10). Initial studies were performed as a batch process,82 where the enolate was generated using sodium hexamethyldisilazide (NaHMDS) at −70 °C, in the presence of an excess of triethylphosphite. Although small-scale lab experiments had shown that the rate of the oxidation was enhanced by the use of pure O2, safety considerations dictated that the O2 concentration in the reactor headspace be kept below 6% on the larger scale process, achieved by use of an air sparge coupled with a nitrogen sweep (3 : 1 volumetric ratio of nitrogen to air), with an oxygen monitor to maintain the O2level at ≤5.5%. Careful control of the enolate formation is required to avoid residual starting material or side-reactions caused by excess base, which was monitored using in-process FT-IR. The resulting process was implemented on a 10 kg batch scale successfully, delivering >7 kg of 16 (71% yield). However, further scale-up of the procedure was deemed not to be feasible on the basis of several considerations, including the extended times required for the aerobic oxidation caused by mass transfer issues, the expense and difficulty of operating in the cryogenic regime (−60 °C), and increased risk associated with reliably purging the headspace at larger scale.
Scheme 10 Synthesis of 6-hydroxybupirone.
The decision was therefore taken to investigate continuous processing methods.45Initial small-scale studies were performed in segmented flow using a microreactor (CPC CYTOS®) with a pre-formed solution of the enolate and oxygen gas as the input streams. A conversion of 85–92% was obtained with a residence time of 5–6 minutes; the short reaction time allowed the reaction temperature to be raised. A higher output can be obtained using a trickle-bed reactor to achieve better gas–liquid mixing. A solution of the enolate was flowed through a 1 inch Pro-Pak-filled column with a counter-current flow of O2 at −37 °C. Operating in continuous mode also allowed for the enolization step to be adapted: an initial mixing of base and 17 at −17 °C and a longer residence time at −35 °C, allowed for complete enolate formation without requiring cryogenic conditions. Further scale-out of the oxidation step was eventually accomplished with four parallel tubular reactors combined within a single cooling jacket, fed by a single oxygen inlet. Under steady-state conditions, the Quadreactor could produce over 100 kg of hydroxybuspirone 16.
In 2015, the Nobel Prize in Medicine was awarded to Youyou Tu for her isolation of the powerful anti-malaria drug artemisinin (19) (‘qinghao su’). Currently, artemisinin and its derivatives are recommended by WHO as the first-line treatment for P. falciparummalaria. Artemisinin is a naturally-occurring compound found in the leaves of the plant Artemisia annua, but the supply from this natural plant is neither sufficient nor reliable enough to meet global demands. To address this issue, a semisynthetic route for producing artemisinin has been implemented on a process scale by Sanofi.83,84 This work has gathered much attention in recent years, not only for its innovation in combining biotechnology with a photochemical reaction, but also serving as an inspiring example of how academia (UC Berkeley), industry (Sanofi, Amyris), non-profit organizations (NRC, Canada, OneWorld Health), as well as funding agencies (WHO, Bill & Melinda Gates Foundation), can form a powerful alliance to deliver innovative solutions to global challenges. The major breakthrough in this remarkable story began with the genetic engineering of Baker’s yeast to produce artemisinic acid, a precursor to the mixed anhydride 20. This is followed by another key transformation, whereby 20reacts with singlet oxygen (1O2) to produce the hydroperoxide intermediate 21, which undergoes rearrangement spontaneously to the artemisinin 19 (Scheme 11). Singlet oxygen is produced photochemically under UV irradiation using tetraphenylporphyrin as a sensitizer. This presents a double challenge for process development: not only does the hazard of handling oxygen present itself, but photochemical reactions are notoriously difficult to scale due to issues with light transmission. In the event, a bespoke semi-batch reactor was designed, wherein a solution of the precursor 20 in dichloromethane and trifluoroacetic acid is circulated with the photocatalyst (ca. 0.05 wt%) at around −10 °C through the photoreactor chamber with air bubbling. The use of air dictates the choice of the chlorinated solvent. The process delivers ca. 370 kg of artemesinin per 600 kg batch of artemesinic acid, and delivered 60 tonnes of 19 in 2014 (a third of the global demand).
Scheme 11 Aerobic oxidation of artemisinic acid to artemisinin 19.
An AstraZeneca team developed a scale-up process for the synthesis of a GSK3β inhibitor used for the treatment of CNS (central nervous system) disorders (Scheme 12). The synthesis route initiates with the lithiation of an imidazole precursor 22, and the addition of the resultant organometallic reactant to the pyrimidine 23. In the following step, the intermediate dihydropyrimidine 24 has to re-aromatise to the desired product 25; this is best achieved by an oxidative dehydrogenation, using air in the presence of a copper catalyst.85 To avoid the risk of organic peroxide formation, a solvent-swap from the THF to acetonitrile following the lithiation/addition sequence is necessary. The aerobic oxidation was subsequently carried out using 5% O2 in N2 to ensure safe operation under batch conditions, delivering ca. 120 g of 25 in a single batch.
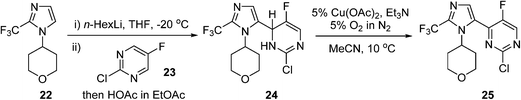
Scheme 12 AstraZeneca route to intermediate 25.
Scale-up of aerobic oxidation of alcohols to aldehydes, mediated by copper(i) salts and nitroxyl radicals, was investigated by the MadOx team.86 Economic (catalytic loadings, additive) and safety (avoiding solvent flashpoint) considerations were taken into account in this work, leading to an optimised batch process that has been carried out on 250 mmol scale on simple test substrates. An application to the synthesis of a pyrimidine intermediate 26 (used for the production of a statin, rosuvastatin) was demonstrated on a 10 mmol scale (Scheme 13). A continuous flow method was subsequently developed using the tube-in-shell membrane reactor described previously (see section 4.5 above). The use of CuI as a catalyst precursor was found to corrode stainless steel components. This prompted the modification of the reactor, where the stainless steel catalyst injection loop was replaced by PTFE. The amount of additive was increased (from 5 to 15 mol%) to prevent precipitation of insoluble Cu species. Using the modified system, the conversion of 1-Boc-3-hydroxyazetidine (27) to the corresponding ketone (28) was demonstrated (Scheme 13): a steady-state yield of ≥98% can be achieved, however, the productivity was not high; only 9 mmol of product was obtained in 3 h.
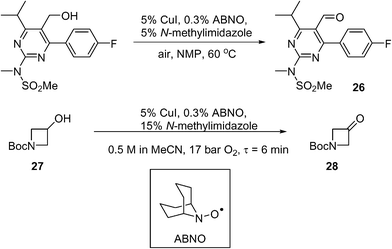
Scheme 13 Cu-catalysed route to a pyrimidine intermediate used in API manufacture.
A similar copper(i)/nitroxyl system for continuous catalytic alcohol oxidation reactions has also been described recently by DSM, to convert the fructose-derived epoxol 29into epoxone 30 (Scheme 14).87 In this process, the catalytic chemistry was implemented in a bespoke 3-D printed ‘zig-zag’ continuous reactor to ensure turbulent flow. Although the reaction rate is independent of O2 pressure, 33 bar of O2was employed to achieve a homogeneous phase. Under these conditions, full conversions of 29 (0.20 M) to 30 can be achieved within 17 min at 100 °C. Economic comparisons between the Cu(OTf) and CuI procedures were highlighted in this work.
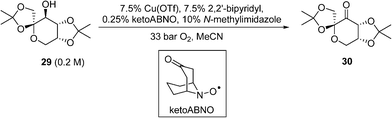
Scheme 14 DSM route for Cu-catalysed aerobic oxidation of an epoxol (29) to epoxolone (30).
Comments are closed