In 1996, world production capacity for styrene was near 19.2 million metric tonnes per year. Dow Chemical is the world’s largest producer with a total capacity of 1.8 million metric tonnes in the USA, Canada, and Europe (1996 figures). The main manufacturing route to styrene is the direct catalytic dehydrogenation of ethylbenzene (above).
The reaction shown above has a heat of reaction of -121 KJ/mol (endothermic). Nearly 65% of all styrene is used to produce polystyrene.
The overall reaction describing the styrene polymerization is:
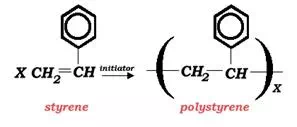
This reaction is carried out in an inert organic solvent environment which provides the reaction medium for this cationic polymerization reaction. The most common solvent used for this reaction is 1,2-dichloroethane (EDC). Other suitable solvents may include carbon tetrachloride, ethyl chloride, methylene dichloride, benzene, toluene, ethylbenzene, or chlorobenzene. The preferred initiator is a mixture of boron trifluoride and water.
The initiator solution is prepared by incorporating 1.5% by weight boron trifluoride gas into the organic solvent (EDC) containing 280 ppm water. This solution is continuously prepared in a holding vessel and will then be injected into the reactor system.
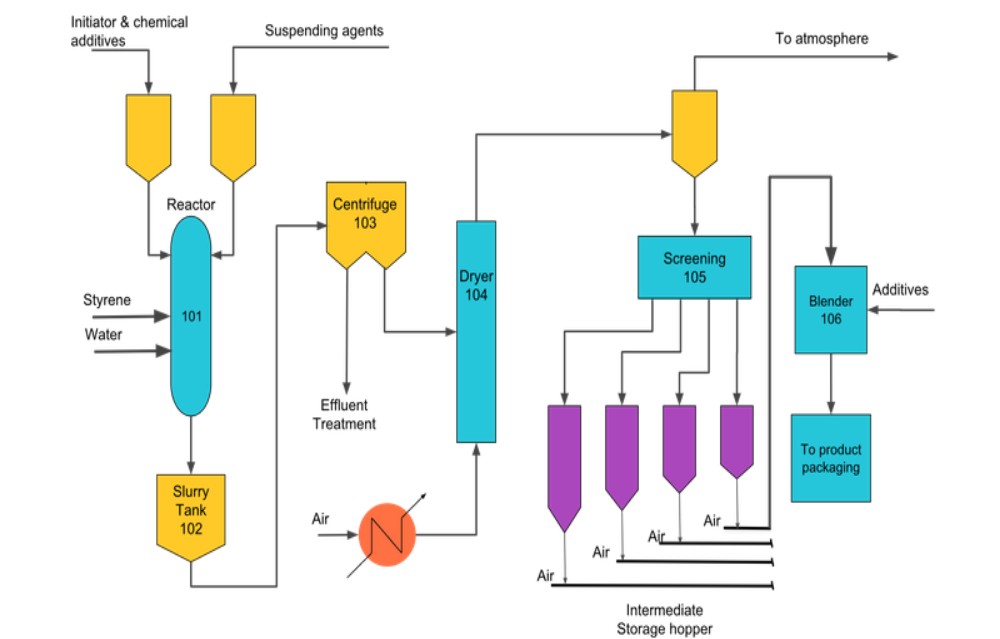
 |
| Figure 1: Block Diagrame for Polystyrene Process |
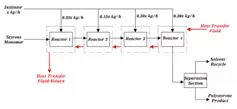
Typical feed to the first reactor would consist of 50 weight percent styrene monomer, 100 ppm water (based on styrene weight), 2000 ppm boron trifluoride (based on styrene weight), with the balance being organic solvent.  The polymerization reaction gives off heat that is carried away from the reactors by jacketing them with a heat transfer fluid. The temperature of the reactants should not vary by more than 15 °C throughout the reactor series.  Temperature control is very important in this reaction because as the reaction temperature increases, the average molecular weight of the polystyrene decreases.  The reaction temperature range is 40-70 °C. Temperature can also be controlled by intermediate shell and tube heat exchangers.
The reaction vessels are typically elongated vessels made of stainless steel. The initiator is introduced as shown below:
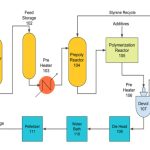
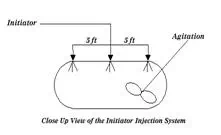 |
| Figure 2: Typical Reactor Overview |