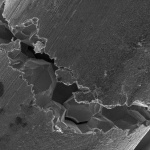
Pitting, just as it sounds, is used to describe the formation of small pits on the surface of a metal or alloy. Pitting is suspected to occur in much the same way crevice corrosion does, but on a flat surface. A small imperfection in the metal is thought to begin the process, then a “snowball” effect takes place. Pitting can go on undetected for extended periods of time, until a failure occurs. A textbook example of pitting would be to subject stainless steel to a chloride containing stream such as seawater. Pitting would overrun the stainless steel in a matter of weeks due to it’s very poor resistance to chlorides, which are notorious for their ability to initiate pitting corrosion. Alloy blends with more than 2% Molybdenum show better resistance to pitting attack. Titanium is usually the material of choice if chlorides are the main corrosion concern. (Pd stabilized forms of Ti are also used for more extreme cases).
Description: Pitting Corrosion
Figure 4: Pitting Corrosion