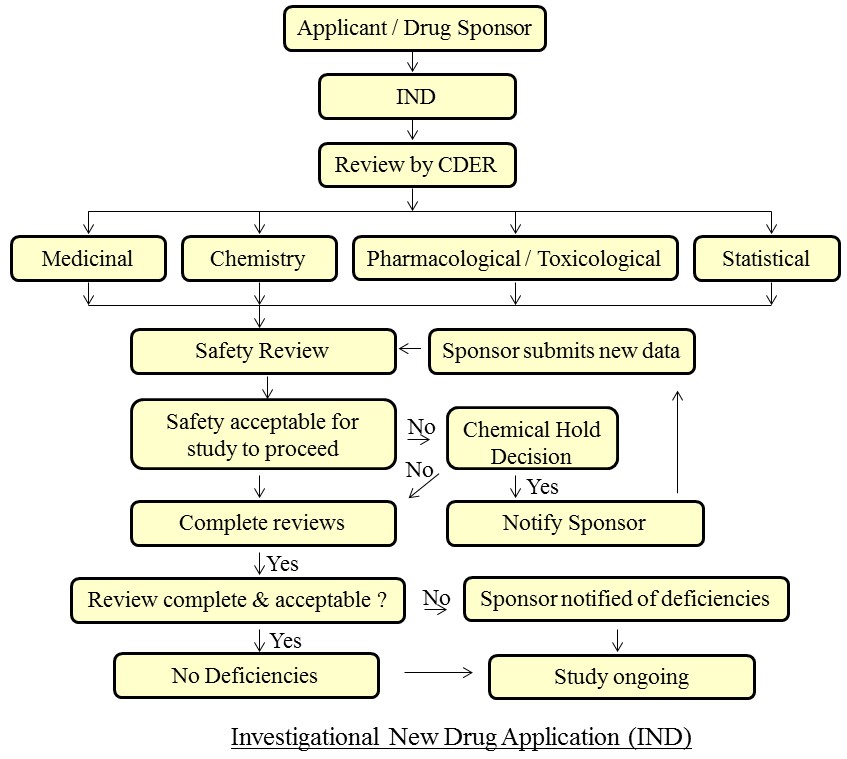
After the above preclinical testing is completed the company then files an IND with the FDA to begin testing the drug in people. The IND will become effective if the FDA does not disprove it within 30 days. The IND will show results of previous experiments, how, where and by whom the new studies will be conducted.  The IND also looks at the chemical structure of the compound, how it works in the body, and any toxic effects found in the animal studies. The IND will also look at how the compound is manufactured.  The IND must be reviewed and approved by the Institutional Review Board where the study will be conducted, and progress reports on clinical trials must be submitted at least annually to the FDA.
Posted inGeneral Concepts